Specialty Crops
Biology and Management of Asiatic Garden Beetle In Indiana Mint Production
Elizabeth Y. Long, Assistant Professor & Specialty Crop Entomology Extension Specialist
If you want to view as pdf, click here
Larvae of the Asiatic garden beetle (AGB) feed on the roots of a wide range of cultivated and wild plant species. In recent years, AGB larval infestations in commercial peppermint and spearmint fields in Indiana have caused significant reductions in vigor and plant stand, and even death of plants in ‘hotspots’ of infestation across mint fields (Figure 1). This extension bulletin provides guidance on how to identify adults and larvae of the Asiatic garden beetle, their life cycle in relation to the seasonal phenology of mint fields, and integrated pest management strategies to monitor and reduce populations of the larvae.
Figure 1. Symptoms of damage by Asiatic garden beetle larvae
in a spearmint field. (Photo Credit: Connor Sturr)
Asiatic garden beetle & other scarab beetles associated with peppermint and spearmint in Indiana
Adult beetles are drab, brown beetles that are 0.35 inches long, roughly the size of a coffee bean. These beetles are in the scarab family, and there are similar-looking beetles that might be confused with Asiatic garden beetles (see E-271, Managing white grubs in turfgrass). Adult AGBs fly at night when temperatures are 65-70 °F. They are attracted to lights and may be captured in light traps. Another common scarab beetle that might be mistaken for Asiatic garden beetle is the masked chafer; however, there are some key morphological differences between the two (Figure 2): 1) when viewed from above, the masked chafer’s head is clearly visible, while the head of AGB appears hidden or obscured from view, 2) the masked chafer has dark coloration on the head between the eyes, giving the appearance of a black “mask”, and 3) the masked chafer’s body is slightly longer (0.45 inches).
Figure 2. Adult masked chafer (MC, top) versus Asiatic garden
beetle (AGB, bottom) on a penny. (Photo Credit: John Obermeyer)
Asiatic garden beetle larvae look like typical scarab larvae, or “white grubs”; they are pale-white, c-shaped, with clearly visible legs and a golden-brown head capsule. The diagnostic character that distinguishes larvae of the Asiatic garden beetle from other scarab larvae, like Japanese beetles or masked chafers, is the presence of mandibular stypes on the head (Figure 3), which look like small, round “cheek pouches”. This is a diagnostic feature; only AGB have these stypes. Additionally, AGB larvae exhibit very active and aggressive behavior compared to larvae of Japanese beetle or masked chafer. For example, when AGB larvae are removed from the soil or handled they quickly try to crawl away and burrow back below the soil surface. Larger AGB larvae are also more likely than other scarab larvae to pinch or bite with their mandibles. Bites are not painful or harmful, but this behavior is helpful for diagnosis.
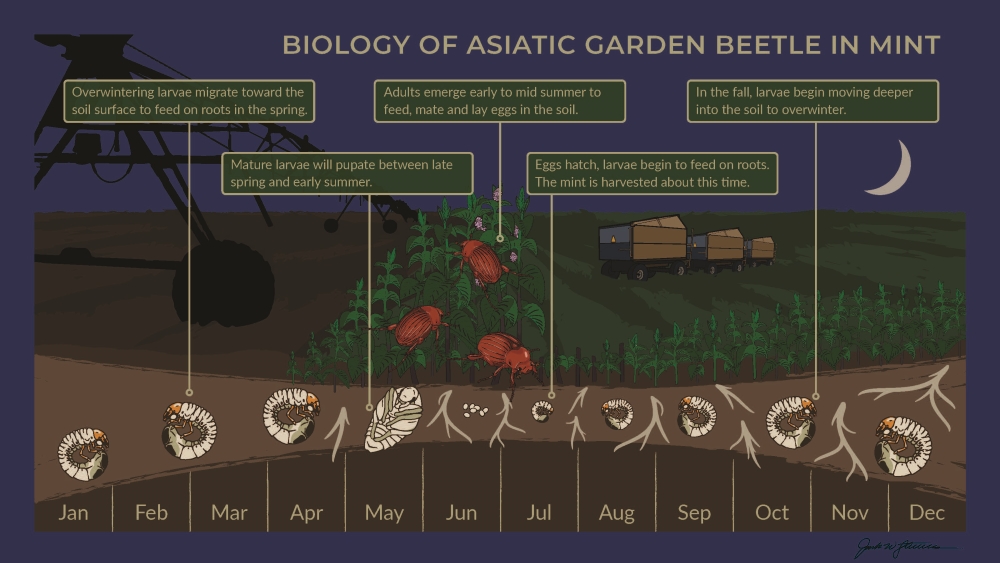
Life Cycle & Seasonal Phenology of AGB in Mint
Adult beetles live for about 4 months, while the larval stage is much longer. AGB exist as larvae in the soil for ~10 months of the year, and during this time they progress through three developmental “stages”, known as “instars”. The three larval instars are progressively larger, and it is the third instar larva that survives the winter in the soil, burrowing deeper in response to cold temperatures in the fall. These larvae then move higher in the soil profile in response to warming soil temperatures in the spring (Figure 4).
Figure 3. Mandibular stypes (yellow circle) are diagnostic
features of Asiatic garden beetle larvae (left), and are not
observed on other scarab larvae, like Japanese beetle larvae
(right). (Photo Credit: John Obermeyer)
Figure 4. The phenology and life cycle of Asiatic garden beetle in commercial mint
production. (Illustration: Jack Stevens)
Third instar AGB larvae begin migrating back towards the soil surface around the same time that mint plants break winter dormancy and begin growing again in the spring (late April). These mature larvae continue to feed on mint roots and begin pupating in the soil from April to June before emerging as adults in late June and early July. Adult activity is typically highest in July and declines steadily through August, but some adults may still be observed until September. During pupation and adult emergence, mint plants continue to grow and by the time adult beetles fly and mate, established mint fields have dense foliage that shades the ground, providing habitat that is preferred by female beetles to lay eggs. Egg-laying typically occurs in early July and after hatching, the first instar larvae begin feeding on fine root hairs, which are critical to the plant for water and nutrient uptake. In the Midwest, mint is typically harvested from late July through August, when AGB larvae are actively feeding on roots. Therefore, root injury by AGB larvae occurs before, during, and after mint harvest, accumulating over time as larvae continue to feed. In November these larvae move deeper in the soil profile to overwinter as third instar larvae. This cumulative summer and fall feeding continues to weaken mint plants after harvest and may reduce overwintering survival of plants, resulting in thinner field stands the following spring.
Damage and Diagnosis
AGB larvae are the damaging life stage of this pest in peppermint and spearmint production; there is no evidence that adults feed on or damage mint foliage. The larvae feed on the fine root hairs (tertiary roots) of mint plants, and when infestations are severe, the tertiary and secondary root hairs may be completely removed by feeding so that only primary root structures remain (Figure 5). Mint plants suffering from damage by AGB larvae may appear wilted, stunted, or with purpling leaves, especially when infestations are severe. Importantly, these symptoms of stress are not unique to AGB larval feeding, so it is always necessary to inspect the soil and plant roots to confirm the presence of AGB prior to taking remedial action (Figure 5).
Figure 5. Mint plants with symptoms of root injury caused by
Asiatic garden beetle (AGB) larvae. Healthy mint plants have
dense, fibrous roots (left), while roots fed on by AGB larvae
have little to no fine roots hairs remaining. (Photo Credit: Connor Sturr)
Monitoring AGB in Mint Fields
Adult beetles are attracted to light, so their activity can be monitored while they fly at night using light traps. Currently, there is no established relationship between the number of adult AGB captured in monitoring traps and levels of larval AGB infestation in mint fields. However, monitoring adult activity, particularly at the beginning of the adult emergence period in June, can help determine the beginning of egg laying activity in fields. Farmers who wish to monitor adults can make a homemade monitoring trap by removing the sides of a plastic gallon-sized milk container, filling with 4 cups of water, adding a few drops of dish soap, and placing a solar-powered pathway light into the opening (Figure 6). This trap can be zip-tied to a landscape stake at waist-height and placed on the edge of the field where it is easy to monitor. Adults that fly into the trap fall into the water and drown. Traps should be checked twice each week so changes in the number of adults captured can be seen. Each time the traps are checked, record the number of beetles, then empty the trap and start again with fresh soapy water. If traps are not checked and emptied regularly, they will not provide useful information about peak adult activity during the current season. Efforts to monitor adult activity should focus initially on fields with a history of AGB infestation.
Figure 6. Homemade light trap consisting of a landscape
stake (A), solar-powered pathway light (B), 4 cups soapy
water (C), and a plastic gallon water jug (D) to monitor adult
Asiatic garden beetle activity at night.
Soil Sampling for AGB Larvae & Damage Threshold
Once peak adult emergence and mating have occurred, typically 2-4 weeks during late June and mid-July, monitoring efforts should be focused on detecting larvae in the soil. The smallest larvae (first instar) may be difficult to detect without careful inspection, but the older larvae (second and third instars) are easily visible in excavated soil. Evidence suggests that 7 soil samples of 0.25 m2 (2.7 ft2) each per 0.2 hectares (0.5 acres), taken with a shovel to a depth of ~4 inches is enough to detect and accurately estimate AGB larval densities in the area. When AGB larval densities exceed 15 ± 2 larvae/0.25 m2 (2.7 ft2), measurable damage begins to occur in the crop, seen as significant reductions in above-ground mint biomass. Again, efforts to monitor AGB larval density should focus on fields with a history of AGB infestation, even focusing on specific areas of fields with previous ‘hotspots’ of infestation and corresponding plant injury.
Simple tools that can make sampling easy and consistent among mint fields include a 0.25 m2 (2.7 ft2) frame, made of PVC or other material, a shovel, a plastic dishpan or bucket, and a soil sieve with a screen size of 0.125-inches, which is big enough to allow soil to pass through, but small enough to catch second and third instar AGB larvae (Figure 7).
Figure 7. Soil sampling for Asiatic garden beetle larvae
using a shovel, a 0.25 m2 (2.7 ft2) PVC frame (left), and a soil
sieve (center) to detect second and third instar larvae (right).
- Place the 0.25 m2 (2.7 ft2) frame on the ground in an area that is suspected of AGB infestation and use it as a guide for the area that you will sample (Figure 7, left).
- Use a shovel to dig up the soil within the frame down to a depth of 4 inches.
- Place the excavated soil in a dish pan or similar container and sort through the soil by hand to find any AGB larvae that may be present (Figure 7, center).
- If a soil sieve is used, sift excavated soil through the sieve and into a container below.
- Remember that larger AGB larvae may be separated from the soil using this technique, but young, first instar larvae may still pass through the sieve.
Managing AGB in Commercial Mint Production
Cultural control strategies. While crop rotation and cultivar/ variety selection are key cultural management strategies for insect pests, there are limitations to these strategies in commercial mint production. Because mint fields typically remain in place for 3-5 years, rotating to another crop the following season to avoid AGB damage is not typically feasible in this crop. To date, there is no research to suggest that mint species (peppermint vs. spearmint) or variety influence the occurrence of AGB infestation or feeding damage by larvae. However, there is evidence to suggest that AGB prefers sandy, welldrained soils. When possible, farmers should avoid planting mint in fields with sandier soils, or in fields where AGB larvae have previously been a problem.
Biological control strategies. Biopesticides, which contain fungi and bacteria, are available and have shown efficacy against AGB larvae in turfgrass and vegetable systems; however, these products have either not been tested against AGB larvae in commercial mint fields, or are not labelled for use in commercial mint oil production systems.
Figure 8. Asiatic garden beetle larvae that have been infected
and killed by insect-parasitic nematodes (top) versus a healthy
larva (bottom). (Photo Credit: Connor Sturr)
Alternatively, biological control agents like insect-parasitic nematodes (IPNs, also referred to as entomopathogenic nematodes) are known to suppress soil-dwelling insects, including AGB larvae. Insect-parasitic nematodes are microscopic roundworms that occur naturally in the soil and only attack insects. There are many different species of IPNs with unique hunting behaviors, but all IPNs attack insects by entering the body through natural openings and releasing symbiotic bacteria that eventually kill and digest the insect (Figure 8). Research with IPNs in a number of cropping systems, including mint, have shown efficacy against AGB larvae; however, most current research indicates that repeated applications of IPNs may be required to maintain population densities that are high enough to suppress AGB larval infestations. Moreover, environmental factors, such as soil moisture, soil texture, and tillage are known to influence the persistence and efficacy of IPNs against soil-insect pests. On-going research in the Entomology Department at Purdue seeks to better understand the population dynamics of IPNs in mint and their potential value as a long-term, sustainable pest management tactic for AGB larval infestations.
Figure 9. Application of insect-parasitic nematodes to the
soil of a post-harvest peppermint field using a tractor boom.
For farmers who are interested in using IPNs to combat soilinsect pests like AGB in mint, it is important to consider 1) the timing of application, so that IPNs are present in the soil when the pest is also present and vulnerable, 2) the method of application, via tractor boom (mesh screens must be removed from nozzles for IPNs to survive the spraying process) (Figure 9) or center pivot irrigation, and 3) soil moisture levels immediately following IPN application, as soil moisture is critical for IPN survival and establishment. Research to evaluate the interaction of these factors and their impact on the efficacy of IPNs against AGB larvae is ongoing.
Chemical control strategies. A variety of conventional (synthetic) and organic insecticides are effective against AGB adults and larvae; however, there are few options labelled for use on mint in Indiana. Systemic insecticides, which are absorbed by foliage and/or roots after application, have efficacy against feeding AGB larvae. The systemic insecticides Coragen eVo and Actara are two options labelled for use on mint.
Insecticide applications should target AGB larvae because they are the damaging life stage and because adults are difficult to reliably manage using insecticides. Since AGB larvae remain in the soil, contacting them with insecticides may require soil-directed applications, followed immediately by irrigation to ensure adequate incorporation into the soil and root zone where larvae are actively feeding. Research indicates that soil-directed applications of systemic insecticides are effective against AGB larvae when used according to the label. For best efficacy, insecticides targeting AGB larvae in mint fields should be applied in mid to late-July, while larvae are small (in the first or second instar stage) and more vulnerable to insecticides. Mint farmers may also consider applying systemic insecticides post-harvest, when plants are most vulnerable to damage. Ultimately, the goal is to kill larvae or reduce their feeding. Preventative treatment of fields with a history of AGB infestation might also be warranted, however, curative treatment of fields that are infested by AGB is likely to be effective, especially when applications are made while AGB larvae are in the first or second instar.
Acknowledgements
This bulletin was developed with support from the Indiana State Department of Agriculture-Specialty Crop Block Grant #A337-21-SCBG-20-102 and Indiana mint farmers.
READ AND FOLLOW ALL LABEL INSTRUCTIONS. THIS INCLUDES DIRECTIONS FOR USE, PRECAUTIONARY STATEMENTS (HAZARDS TO HUMANS, DOMESTIC ANIMALS, AND ENDANGERED SPECIES), ENVIRONMENTAL HAZARDS, RATES OF APPLICATION, NUMBER OF APPLICATIONS, REENTRY INTERVALS, HARVEST RESTRICTIONS, STORAGE AND DISPOSAL, AND ANY SPECIFIC WARNINGS AND/OR PRECAUTIONS FOR SAFE HANDLING OF THE PESTICIDE.
August 2024

It is the policy of the Purdue University Cooperative Extension Service that all persons have equal opportunity and access to its educational programs, services, activities, and facilities without regard to race, religion, color, sex, age, national origin or ancestry, marital status, parental status, sexual orientation, disability or status as a veteran. Purdue University is an Affirmative Action institution. This material may be available in alternative formats.
This work is supported in part by Extension Implementation Grant 2021-70006-35390/ IND90001518G-1027053 from the USDA National Institute of Food and Agriculture and NCR SARE Award GNC20-311.
765-494-8491
www.extension.purdue.edu
Order or download materials from www.the-education-store.com