Specialty Crops
MANAGEMENT STRATEGIES FOR COMMON ARTHROPOD PESTS OF GREENHOUSE HEMP IN INDIANA
Zachary Serber and Elizabeth Y. Long, Department of Entomology
If you want to view as pdf, click here
Indoor hemp production presents unique challenges and opportunities for Indiana growers. On one hand, greenhouse environments provide growers with the ability to grow hemp year round while maintaining optimal day-length, temperature, and nutrient levels for hemp plants. On the other hand, greenhouse environments have an associated suite of insect pests that can be especially challenging to manage, particularly in the case of hemp, which has very few insecticides registered for use. Despite this limitation, there are opportunities to develop and optimize biological control strategies as a sustainable alternative or complement to insecticide-based management of arthropod pests of indoor hemp.
This bulletin provides an overview of common insect and mite pests of hemp in greenhouse environments, including their biology, signs and symptoms of damage, and management strategies to prevent pest population outbreaks.
CANNABIS APHID (Phorodon cannabis)
The cannabis aphid (Figure 1) is a small, soft-bodied insect with a piercing-sucking feeding strategy. The cannabis aphid feeds by inserting its straw-like mouthparts into plant stems and leaves to extract plant phloem. Adult cannabis aphids are 1.8–2.7 mm long, visible to the naked eye, and may be winged or wingless. Nymphs are wingless and look like miniature versions of the adult. These aphids feed exclusively on Cannabis and have no known alternative host plants. Cannabis aphids can vary significantly in color throughout the season, ranging from pale pink or green to brown. Cannabis aphids have long antennae (1.1–2.2 mm), and two rear-facing tubes, called cornicles, at the end of the abdomen. The cannabis aphid is not the only aphid you may see on hemp plants; other aphids such as the green peach aphid have also been observed. Close investigation of the antennae and cornicles is required to distinguish cannabis aphid from other aphids. Relative to other aphids, the cannabis aphid has long cornicles that are nearly ⅓ the length of the body.
Figure 1. Cannabis aphids on a hemp leaf. (Photo credit: John Obermeyer, Purdue Entomology)
These insects can be found in indoor and outdoor hemp systems early in the season, and in greenhouse production cannabis aphids have the potential to be year-round pests. The biology and life cycle of the cannabis aphid is not well studied; however, it is likely similar to that of other aphids: females reproduce asexually and give birth to live young (nymphs) that develop for 3 to 4 weeks. Adults typically live for ~1 month and there are many overlapping generations each year.
Damage
Cannabis aphid feeding does not cause physical damage to the surface of leaves or buds. Rather, cannabis aphids cause reductions in plant vigor by removing plant nutrients, leading to yellowing, wilting, and stunted growth. Additionally, as cannabis aphids feed, they excrete a sugary, liquid waste called honeydew that accumulates on plant surfaces, giving them a shiny or wet appearance. At high levels of aphid infestation, this honeydew contributes to the development of sooty mold on leaves and buds, which can reduce yields, limit photosynthesis, and further reduce plant growth.
Monitoring and Scouting
Cannabis aphid populations can be monitored on hemp plants by inspecting buds, stems, and the undersides of leaves for aphids. Because populations can grow rapidly, it is important to scout hemp plants throughout the greenhouse (edge and center of benches, near/far from walkways and doorways) frequently, at least twice a week. The presence of papery-white cast aphid “skins” (shed exoskeletons) on plant leaves, stems, or the soil, and yellowing or wilting leaves, as well as honeydew and sooty mold are key signs and symptoms of an aphid infestation.
GREENHOUSE WHITEFLY (Trialeurodes vaporariorum)
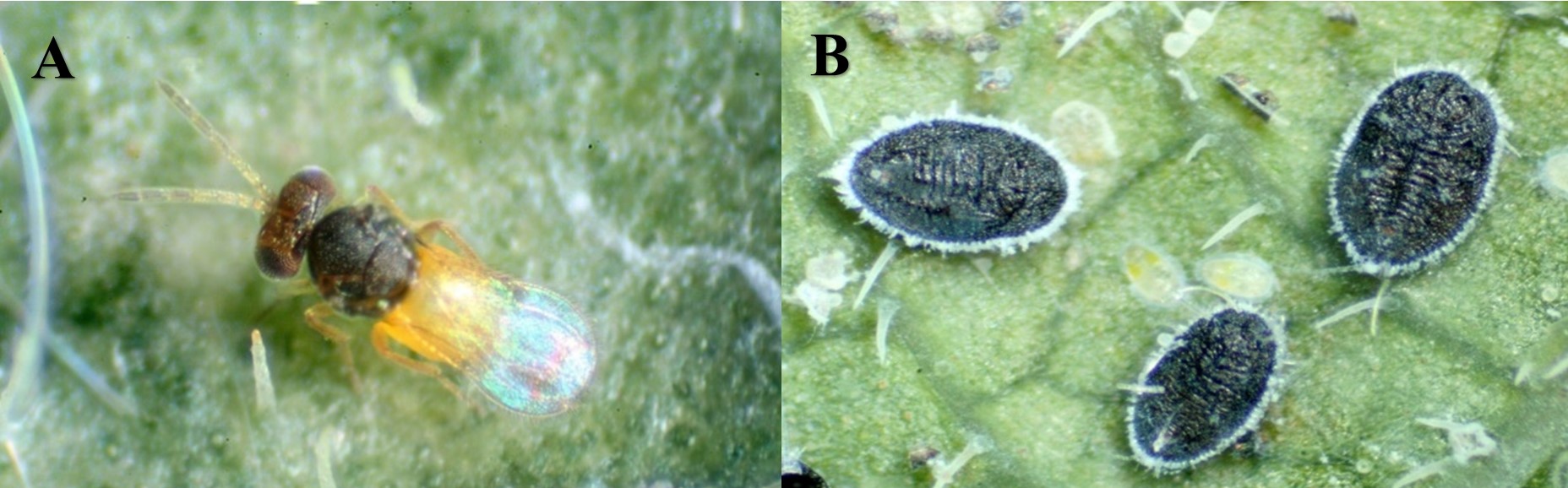
The greenhouse whitefly is a small (1.5-2.5mm), moth-like insect with powdery-white wings and short antennae (Figure 2A). The nymphal stages of the greenhouse whitefly look very different from adults: nymphs are wingless and look like flattened, semi-transparent ovals located on the undersides of leaves (Figure 2B). Whitefly nymphs may also be mistaken for scales, leaf spots, or even the eggs of other insects.
Figure 2. Adult whitefly on a hemp leaf (A), and third/fourth-instar whitefly nymphs with emerging whitefly adult (B). (Photo Credits: Zachary Serber and John Obermeyer, Purdue Entomology) *Pictures not to scale.
Greenhouse whiteflies can survive and reproduce year round in controlled environments when suitable host plants are available. Adult whiteflies live for 4 to 6 weeks, and the entire life cycle (egg to adult) can be completed in 14-28 days, with development occurring more rapidly in warmer temperatures and slowing in cooler temperatures. Female whiteflies lay 200-400 eggs during their lifespan, and eggs are laid in circular clusters on the undersides of leaves. Eggs hatch after 5-10 days into first instar nymphs, called crawlers. Crawlers move only a short distance from the hatch site before settling permanently in one spot to feed. All nymphal stages that follow the crawler stage are sedentary and are typically found on the undersides of leaves. After the crawler stage, the greenhouse whitefly progresses through three instars (nymphal growth stages), molting every 2-4 days and growing larger from each stage to the next. After the final instar, nymphs enter a pupal (pre-adult) stage that lasts for ~7 days. Greenhouse whiteflies often exhibit overlapping generations in controlled environments, such that all life stages may be present at once. Whitefly infestations on hemp have been characterized as light, moderate, and heavy when 1-5, 5-10, and 10-20 adult whiteflies, are detected per plant, respectively.
Damage
Hemp plants that are infested with whiteflies may not present many symptoms until whiteflies reach outbreak levels. The most obvious sign of whitefly infestation are “bursts” of adult whiteflies dispersing from plants during watering or handling. Heavily-infested plants may exhibit symptoms that are similar to those caused by aphid feeding: yellowing, drooping, and wilting leaves, as well as the presence of honeydew and sooty mold on leaf surfaces.
Monitoring and Scouting
As whitefly infestations have few early symptoms, scouting and monitoring hemp plants for nymphs and adults are critical in preventing outbreaks. Quick surveys of whiteflies can be performed by gently tapping or shaking plants to see if adults fly away. However, yellow sticky cards are the most reliable monitoring tool to gauge whitefly populations in the greenhouse. The yellow color of the cards attracts adult whiteflies, and when they land, they are trapped by the sticky glue on the card surface. This passive monitoring strategy makes it easy to count the number of adults captured and track the number of captures over time. Sticky cards should be checked weekly so that sudden increases in the population do not go unnoticed. Currently, it is unclear if whitefly infestations cause economic losses to hemp, so there is no formal action threshold for whiteflies on hemp at this time.
HEMP RUSSET MITE (Aculops cannibicola)
Hemp russet mites (Figure 3) are one of the most difficult pests to manage on hemp. These microscopic (0.16-0.21 mm) eriophyid mites have a piercing-sucking feeding strategy and are not visible to the naked eye, and even with high magnification, they can be difficult to detect on hemp leaves. Hemp russet mites have a tubular body shape and differ from other mites in having two pairs of legs rather than four pairs of legs.
Figure 3. Magnified images of hemp russet mites on a hemp leaf.
(Photo credit: John Obermeyer, Purdue Entomology)
Hemp russet mites can survive and reproduce year round on hemp grown in controlled environments. These mites only feed on Cannabis sativa and have no known alternative host plants. Although the hemp russet mite has not been studied as extensively as other mites, its biology and life cycle are likely similar to that of russet mite species that feed on other herbaceous plants, such as tomato russet mite. Russet mites lay clear, round eggs on the undersides of leaves that typically hatch within 1-3 days. Most russet mites have two nymphal (immature) stages before molting into the adult stage. The life cycle can be completed in 8-15 days, with faster development in warmer temperatures and slower development in cooler temperatures.
Damage
Hemp russet mites feed by piercing plant cells on the surface layer of leaves to extract nutrients. When these mites are at low densities, there may be no visible symptoms of damage. However, at high levels of infestation leaf edges curl upward and become dull in color, called “russeting,” (Figure 4) and some areas on leaves may exhibit brown spots or speckling as a result of physical damage to cells on the leaf surface.
Figure 4. “Russeting” of hemp leaves caused by hemp russet mite feeding. (Photo credit: McPartland, J.M. and Hillig, K.W. 2003. The hemp russet mite. Journal of Industrial Hemp, 8:2, 109, DOI: 10.1300/J237v08n02_10)
Monitoring and Scouting
Management of this pest is especially difficult because hemp russet mite is not visible to the naked eye. Therefore, common strategies to detect mites, like the leaf-shake test over a white paper plate or piece of paper, are not useful. Instead, it is very important to scout hemp plants, especially cuttings or clones that are bought or sold, for symptoms of russet mite damage, including leaf russeting, brown spots or speckles, and upward leaf curling. If hemp russet mites are suspected on plants, leaf samples can be submitted to the Purdue Plant and Pest Diagnostic Laboratory (https://ag.purdue.edu/btny/ppdl/Pages/default.aspx) to confirm detection on hemp.
TWO-SPOTTED SPIDER MITE (Tetranychus urticae)
Two-spotted spider mites (Figure 5) are small mites (0.4 mm in length) with a round body shape and a piercing-sucking feeding strategy. Spider mites are barely visible to the naked eye, so a hand lens or magnifying glass may be necessary to see them. Spider mites may appear translucent-beige or greenish-yellow in color; however, they can also appear dark red in color during the winter. The “two-spotted” descriptor in the name refers to the presence of two dark-colored spots on the abdomen. These spots are stored waste products in the abdomen that are visible through the transparent exoskeleton of the mite. However, these two spots may not be apparent on newly-emerged mites until they have fed on plants.
Figure 5. Two-spotted spider mite adult (bottom left), nymph (top), and egg (bottom right).
(Photo credit: John Obermeyer, Purdue Entomology)
The life cycle of two-spotted spider mites can be completed in ~12-15 days in controlled environments, and when conditions are optimal one generation may be completed in 5-10 days. Spider mite eggs are tiny, round and translucent to white in color, and females produce 50-100 eggs during their lifetime. Eggs are attached to the undersides of leaves with a small piece of silk and hatch after 1-3 days into nymphs that have only six legs. Nymphs begin feeding immediately and progress through three instars before reaching the adult stage. Second and third instar nymphs have 8 legs like the adults. Given the rapid life cycle, there are many overlapping generations of spider mites in greenhouse environments each season.
Damage
Two-spotted spider mites pierce plant cells on the surface layer of leaves to feed on cell contents that ooze out. Given their small size, two-spotted spider mite infestations are often overlooked until they have already reached damaging levels. If infestations are detected early, plants may recover without significant damage to the foliage or flowers.
Hemp plants that are heavily infested with two-spotted spider mites exhibit leaf yellowing, brown or yellow stippling on leaves, and the presence of silk webbing on foliage and flowers (Figure 6).The webbing serves as a protected environment and a means of movement for spider mites, and presence of webbing on buds reduces the unit price and may even result in rejection by consumers entirely. Two-spotted spider mites may also infest hemp buds and create webbing after harvest while buds are still drying. As a result, two-spotted spider mite is often considered the most damaging arthropod pest of indoor-grown hemp.
Figure 6. Webbing and leaf yellowing that are characteristic of two-spotted spider mite presence on plants.
(Photo credit: John Obermeyer, Purdue Entomology)
Monitoring and Scouting
Manually inspect hemp plants for two-spotted spider mites by using a 10-15x magnification hand lens to detect adults and nymphs on the undersides of leaves. Because these mites are more visible to the naked eye, scouting can also be performed by shaking parts of the plant over a white piece of paper or paper plate. Also look for signs, including webbing and eggs, and symptoms such as leaf yellowing and stippling. Spider mites can be both pre-harvest and post-harvest pests. As such, it is important to scout hemp plants regularly and throughout the greenhouse to detect two-spotted spider mite populations before they reach outbreak levels.
MANAGEMENT STRATEGIES FOR ARTHROPOD PESTS OF INDOOR HEMP
While insect and mite pests in other systems are managed with various insecticides, these options are limited for use on hemp. Therefore, an integrated approach is necessary for effective management of these pests in indoor hemp production.
Cultural Control
Sanitation practices and plant isolation are critical strategies for indoor growers of hemp to reduce infestation and spread of arthropod pests. It is often easier to keep pests out of a greenhouse than it is to remove them once they enter and become established. As such, practices should be implemented to prevent pests from entering greenhouses and to prevent the movement of pests between production bays. By combining sanitation with preventative biological and cultural control strategies, growers may successfully keep pest populations below damaging levels.
For example, indoor-grown plants may be infested with insect pests via the introduction of live plants or cuttings that are already infested. Insect pests may also be accidently transported by workers that have handled infested plants. To prevent the spread and establishment of insect and mite pests, strict quarantine steps should be employed in greenhouse spaces. The best practice is to isolate new plants and cuttings from outside sources or other greenhouses from the main growing area until new plants are determined to be pest free. If infestations are detected, growers should isolate and possibly even destroy affected plants and then quarantine infested bays. Pests like mites can also be transferred between areas by workers. As such, growers should limit and document movement in and out of these spaces to prevent transfer of insects from contaminated bays to clean bays.
Efforts should also be made to eliminate weeds both inside and around greenhouse spaces. Weeds inside greenhouse spaces may provide alternative food resources or refugia for insect pests and allow infestations to persist over successive growing seasons. Weeds outside or nearby greenhouse spaces, especially those near entrances or ventilation systems, may facilitate the entry of arthropod pests into protected areas. Examples of common weeds that can harbor hemp pests, like two-spotted spider mites and whiteflies, are chickweed, henbit, pokeweed, velvetleaf, vetch, and wild mustard.
Sanitation and cultural control efforts should not end once plants are harvested. After harvest, growers should clean indoor production spaces and remove any residual plant material (weeds, fallen leaves, etc.). If possible, previously-infested greenhouse bays should be left fallow for a period between harvest and planting (i.e., no “green bridge”) to eliminate food sources and break the life cycle of any remaining pest populations.
Biological Control
While sanitation and cultural control practices focus on preventing infestations, they may still occur. In these cases, biological control, including the use of commercially-available predators, parasitoids, and pathogens, may help maintain pest populations below damaging levels. Predatory insects and parasitoids can be purchased commercially from vendors, which are listed here: https://entomology.ca.uky.edu/ef125.
Generalist predatory insects, such as lady beetles, lacewing larvae, and minute pirate bugs, feed on a variety of insects and insect life stages, and so can be effective against greenhouse pests such as aphids, whitefly nymphs, and adult mites. Releasing a combination of generalist natural enemies may sometimes enhance pest suppression; however, care must be taken because some generalist predators will also attack and feed on other predatory insects, reducing the overall efficacy of pest suppression. For example, generalist lady beetles may eat minute pirate bugs, and even small lacewing larvae, such that these predators are no longer available to attack focal pests.
There are also specialist predatory insects and parasitoids, which only attack specific groups or types of insect pests. For example, the lady beetle, Delphastus catalinae, and the parasitoid wasp, Encarsia formosa, are specialist insects that feed exclusively on whiteflies. Generalist and specialist natural enemies each have benefits and shortcomings. For example, although specialists only consume certain pests and are unlikely to consume other predators, they are not effective in suppressing populations of multiple pests that may be present on indoor hemp at the same time. Meanwhile, generalists may be more likely to eat other predatory insects, but may be able to suppress several different pests at once, allowing for more cost-effective management.
APHID AND WHITEFLY PARASITOIDS
There are several commercially available parasitoids that attack cannabis aphids: Aphidius colemani (Figure 7A), Aphidius ervi (Figure 7B), and Aphidius matricariae. These parasitoids are tiny, stingless wasps that search out aphids to sting and lay eggs inside. The eggs hatch into larvae that eat the aphid from the inside, eventually killing it. Aphids that have been attacked by parasitoid wasps eventually become sessile and are referred to as “mummies” (Figure 8). Aphid mummies appear dry and bloated and may be gold, brown, or black in color.
Figure 7. Whitefly parasitoids Aphidius colemani (A) and Aphidius ervi (B). (Photo credit: David Cappaert, Bugwood.org; Evergreen growers supply©. Note: images not to scale.)
Figure 8. Aphid mummies on a hemp leaf. (Photo Credit: Whitney Cranshaw, Bugwood.org.)
Whitefly parasitoids, like Encarsia formosa (Figure 9A), are similar to aphid parasitoids in their life cycle and attack strategy: they seek out whitefly nymphs to sting and lay eggs inside. Whitefly parasitoids typically attack older (larger) whitefly nymphs. Nymphs that have been stung by a parasitoid eventually become sessile and turn black (Figure 9B). Parasitoid wasps that attack whitefly nymphs may complement pest suppression by natural enemies that attack other life stages of the whitefly, such as Delphastus catalinae, a predatory lady beetle that feeds on whitefly eggs and younger (smaller) whitefly nymphs.
Figure 9. Whitefly parasitoid, Encarsia formosa (A), and parasitized whitefly nymphs (B). (Photo Credit: David Cappaert, Bugwood.org. Note: images not to scale.)
Parasitoid wasps can be very effective at managing aphids and whiteflies at low levels of infestation. However, parasitoids are best used as a preventative measure and are not a rescue treatment. Parasitoids cannot eliminate pests after populations have reached high levels of infestation.
PREDATORY MITES
Several species of predatory mites can be used to manage two-spotted spider mites and russet mites in controlled environments; however, not all are generalist predators of other pest mites. For example, the predatory mite Phytoseiulus persimilis (Figure 10A) only feeds on web-spinning spider mites in the subfamily Tetranychinae, which includes important pests like the two-spotted spider mite. In contrast, predatory mites like Amblyseius andersoni and Neoseiulus californicus (Figure 10B) attack several species of plant-feeding mites, including spider mites and russet mites. These predatory mites may also feed on thrips and pollen, allowing them to persist in greenhouse environments when pest mite populations are low. In general, predatory mites attack multiple life stages (eggs, nymphs, and adults) of pest mites, but this may vary depending on the focal species of predatory mite. Research-based information on the efficacy of predatory mites against pest mites on hemp is limited currently, especially for hemp russet mite. Until we have more information, it is important to properly identify pest mites detected on hemp plants and consult with commercial vendors and your local extension specialist to develop a management plan for mite pests on hemp.
Figure 10. (A) Female (left) and immature (right) predatory Phytoseiulus persimilis mites. Photo: Merritt Singleton, University of Wisconsin. (B) A predatory Neoseiulus californicus mite eating a spider mite. (Photo Credits: Weintraub P, (Amiel) Recht E, Lilach L, Mondaca, Harari A, Diaz B, and Bennison J. 2017. Arthropod pest management in organic vegetable greenhouses. Journal of Integrated Pest Management)
Chemical Control
If cultural and biological control strategies fail to manage arthropod pest populations in hemp, insecticide applications may be considered. The list of registered pesticides for use on hemp in Indiana can be viewed on the Office of the Indiana State Chemist’s website.
READ AND FOLLOW ALL LABEL INSTRUCTIONS. THIS INCLUDES DIRECTIONS FOR USE, PRECAUTIONARY STATEMENTS (HAZARDS TO HUMANS, DOMESTIC ANIMALS, AND ENDANGERED SPECIES), ENVIRONMENTAL HAZARDS, RATES OF APPLICATION, NUMBER OF APPLICATIONS, REENTRY INTERVALS, HARVEST RESTRICTIONS, STORAGE AND DISPOSAL, AND ANY SPECIFIC WARNINGS AND/OR PRECAUTIONS FOR SAFE HANDLING OF THE PESTICIDE.
December 2021

It is the policy of the Purdue University Cooperative Extension Service that all persons have equal opportunity and access to its educational programs, services, activities, and facilities without regard to race, religion, color, sex, age, national origin or ancestry, marital status, parental status, sexual orientation, disability or status as a veteran. Purdue University is an Affirmative Action institution. This material may be available in alternative formats.
This work is supported in part by Extension Implementation Grant 2021-70006-35390/ IND90001518G-1027053 from the USDA National Institute of Food and Agriculture.
765-494-8491
www.extension.purdue.edu
Order or download materials from www.the-education-store.com