Varroa mites are just one of many stressors that can harm honey bees.
Biology and Control of Varroa Mites in Bee Hives
Greg Hunt and Krispn Given,
Purdue Entomology
If you want to view as pdf, click here
The varroa mite (Varroa destructor) was originally a parasite of the Asian honey bee (Apis cerana) but gained the ability to infest the European honey bees commonly used for beekeeping (Apis mellifera). The tracheal mite (Acarapis woodii) was first described as the causal agent of Isle of Wight disease in the United Kingdom in the early 20th century.
These mite species affected hives in Europe and Asia for many years, but beekeepers and researchers only noticed these mite species in the United States around 1990. Tracheal mites were the first to cause heavy winter losses of bee hives in the United States. However, surveys conducted from 2010 to 2016 show that more colony losses in North America and Europe are linked to varroa mites than any other factor.
Varroa mites are ectoparasites, which means that they live on the outside of bees and feed off the bees’ internal fluids. The mites use their mouthparts to pierce the bees’ bodies.
In contrast, tracheal mites are microscopic parasites that live in the tracheae (or breathing tubes) inside of bees. Tracheal mites partly clog the breathing passages and can seriously affect bee health.
Most bee stocks have become sufficiently resistant to tracheal mites that the bees do not require tracheal mite treatments to prevent colony losses. It should also be noted that some fumigants used to control varroa mites (such as thymol and formic acid) also kill tracheal mites. If less than 5 percent of the bees in your hive have tracheal mites, then the hive should be able to survive the winter, which is when these mites stress bees the most.
To detect tracheal mites one must slice the thorax of each bee into pieces, stain the tissue, and examine it under a microscope at 400 times magnification. Beekeepers rarely check for tracheal mites or use treatments to control them, because resistant bees are now common. Since tracheal mites are a less serious problem, the rest of this publication focuses on varroa mites. We will examine the biology of varroa mites, the problems they can cause, and how to monitor and control them.
Varroa mites look like ticks on honey bees (Figure 1). The mites appear as coppery brown (or red-brown) discs that are about 1.5 mm wide and they are quite mobile. The mites pierce the bee’s exterior to feed. Varroa mites reproduce by exploiting the honey bee life cycle. Honey bee larvae develop inside the brood cells in the wax comb. When the larvae are ready to transform from larvae to adults (metamorphosis), adult worker bees will seal larvae (now pupae) inside individual brood cells. The larvae will pupate and emerge as an adult.
Figure 1. This photo shows a tiny varroa mite (the small, coppery brown disk) on the back of the honey bee.
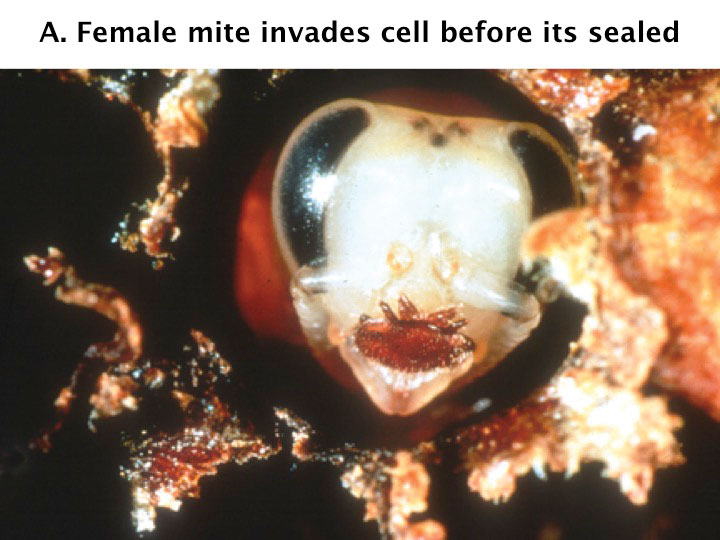
Female varroa mites enter the bee hive’s brood cells to reproduce. The mites use the same chemical cues that bee larvae produce that signal worker bees to seal the cell (Figure 2). The varroa female punctures the underside of the abdomen of the pupa to feed on the fat body cells before laying its first egg.
Like honey bees, the male mite develops from an unfertilized egg. After about 72 hours in the cell, the female mite lays an egg that becomes a haploid male. A haploid individual has only one set of parental chromosomes. The female mite continues to lay about one egg per day. These eggs develop into daughters that must mature and mate with the male before the bee emerges from the brood cell. Only mature female mites will survive after leaving the brood cells. The immature mites and males die soon after the adult bee emerges.
Varroa prefer to enter drone cells but can also reproduce in the worker cells of European honey bees. In Asian honey bee colonies, the mites are only able to reproduce in drone cells. Varroa mites are now so common that they can be found in nearly every hive in the United States.
Figure 2a. An adult female mite in a honey bee brood cell before it is sealed.
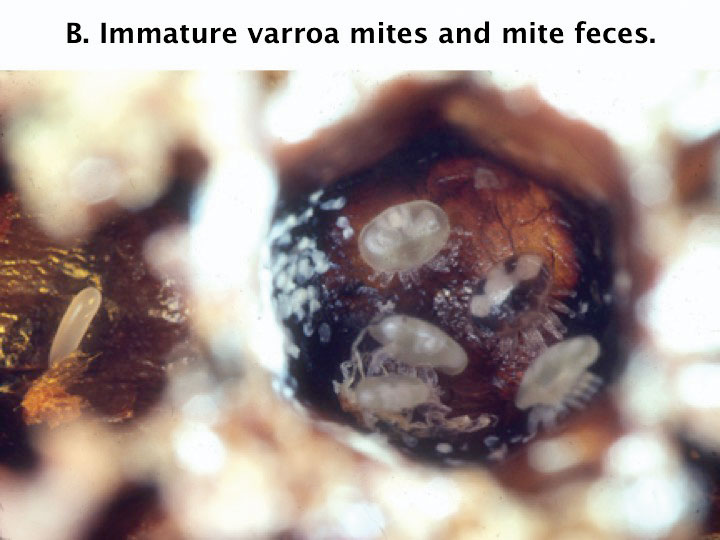
Figure 2b. The pupa has been removed fro this brood cell to reveal mites and a white patch of mite feces. Mites gather to mate in the fecal patch.
Heavily infested bee hives can look very healthy and produce good honey crops, only to dwindle and die during fall or winter. Colonies that have large bee populations produce a lot of brood, which allows mite populations to grow.
The varroa mite population in the hive usually peaks in early fall. This is when the bees are getting ready to produce the last batch of worker bees for the year. These “winter bees” do not need to make protein-rich royal jelly to feed larvae so the pollen that the young bees eat is converted into vitellogenin, the protein that is stored in their fat bodies and blood (hemolymph), which helps them live all winter long. Winter bees also use vitellogenin to make royal jelly that they feed to larvae as the queen starts laying eggs even before the flowers resume blooming in the spring.
Winter bees also need to produce heat to incubate the brood at about 93°F when egg laying resumes (usually about February). It is critical for the hive to have a healthy winter bee population for colony survival. Varroa mite feeding causes vitellogenin levels to drop. The feeding mites can also transmit viral diseases, which drastically reduce the bees’ lifespans.
The symptoms of “parasitic mite syndrome” are common in hives in the fall, but can also occur earlier in the growing season or during winter (Figure 3). Typical symptoms include evidence of a number of virus and brood diseases, but sometimes the colony just dwindles with no obvious symptoms as the sick foraging bees simply do not to return to the hive. A dwindling adult population is also symptomatic of high tracheal mite infestations.
Figure 3. This photo shows symptoms of parasitic mite syndrome. Note the two worker bees with deformed wings and a mite on the bee in the center. The brood pattern is spotty because bees have removed dead larvae.
It is important for beekeepers to have an idea of the mite populations in their hives. It is easy to overlook the presence of varroa during a casual hive inspection. Beekeepers usually do not notice the mites on bees until there are a lot of mites in the hive.
Since varroa mites prefer drone brood, you may find mites by opening sealed drone cells. Bees often build drone comb between the hive boxes, so splitting the boxes apart may open drone cells for inspection. Look for the coppery brown mites on the white pupae. If many cells look infested, you may have a problem.The most sensitive method for detecting mites is to sample them with a “sticky board.” Some hive boxes have screened bottom boards and a plastic sheet can be inserted after spraying it with vegetable oil. Or, you can construct a sticky board of three-eighths-inch-thick wooden frame with a screen stapled to it. Put contact paper on the bottom, spray vegetable oil on it, and insert it in the colony entrance (Figure 4). The screen prevents the bees from removing mites from the sheet.
Figure 4. This beekeeper is inserting a homemade sticky board sampling device into a hive to monitor varroa mite populations.
It is also possible to remove mites from adult bees for sampling. The “ether roll” method consists of scooping up half a cup of bees (about 300 workers — be careful not to collect the queen) into a jar with a screened lid. Spray engine starter fluid into the jar, which will kill the bees and dislodge the mites. The mites stick to the glass.
Another popular method to check for mites is to again use a jar with a screened lid, then put half a cup of bees (about 300 bees) in the jar. Instead of killing the bees, coat them liberally with powdered sugar. Let the jar sit for two minutes and then shake the mites onto a white surface. Keep shaking until few mites are falling. Spraying water on to the powdered sugar makes the mites easier to see. It is best to take three samples if you want a more accurate measure of the mite population on the bees.
When the mite population reaches a particular level, apply a treatment to kill the mites. Treatment thresholds vary with the season.
For example, if the ether roll or powdered sugar tests reveal 15 mites on about 300 bees, your hive has a 5 percent infestation rate. A spring infestation of 3 percent or a late-season infestation of 5-10 percent is unacceptably high in the Midwest. For the sticky board test, count the mites that fell on the board for a 24-hour period. Treat for mites if you find 10 mites on the board early in the spring, or 50 mites on the board late in the season.
Table 1 shows most of the products that are registered to control varroa as of January 2017. Sucrose octanoate is also registered, but we do not recommend it.
These control agents can be divided into three categories:
- Synthetic miticides (such as fluvalinate, coumaphos and amitraz).
- Naturally occurring molecules (such as formic and oxalic acids).
- Products derived from plant volatiles (such as
thymol, hop beta acids, and other plant oils).
Beekeepers administer synthetic miticides by hanging a miticide strip between the combs. For the other treatments (except dribbling oxalic acid onto clusters of bees), beekeepers administer them as vapors (or fumigants) within the hive. It is important to follow product directions and pay careful attention to temperature and hive ventilation.
Many U.S. mite populations have become resistant to fluvalinate and coumaphos, but these products may still be effective again if they have not been used for several years. Amitraz is a good choice for commercial beekeepers who have large numbers of hives and need a reliable and easy-to-apply control method. The other products listed will provide adequate control when used properly, but may require repeated applications especially when brood is present.
When they can access brood cells, most of the varroa mites in a hive will be sealed inside the brood cells, so the miticide will not kill them. Worker brood is sealed for about two weeks before the adult bees emerge. Be sure to wear protective gloves and follow label instructions. To avoid contaminating honey, you should not apply some of these products when honey supers are on the hive.
Table 1. Some products registered by the EPA to control varroa mites.
| Registration Number | Product Name | Active Ingredient |
|---|---|---|
| 2724-406 | Zoecon RF-318 Apistan Strip | fluvalinate (10.25%) |
| 61671-3 | For-Mite (fumigant) | formic acid (65.9%) |
| 73291-1 | Api Life Var (fumigant) | thymol (74.09%), oil of eucalyptus (16%), menthol (3.73%) |
| 75710-2 | Mite-Away Quick Strips (fumigant) | formic acid (46.7%) |
| 79671-1 | Apiguard (fumigant) | thymol (25%) |
| 83623-2 | Hopguard II (fumigant) | hop beta acids resin (16%) |
| 87243-1 | Apivar (strip) | amitraz (3.33%) |
| 91266-1 | Oxalic Acid Dihydrate (fumigant or liquid dribble onto bees) | oxalic acid (100%) |
| 11556-138 | Checkmite+ Bee Hive Pest Control Strip | coumaphos (10%) |
Figure 5. Bee hive.
Honey bees are gaining some ability to fight back against varroa mites. The two most important traits that allow bees to control mites are both behavioral responses. The first trait involves uncapping and removing mite-infested pupae. Bees with this trait have what is called varroa-sensitive hygiene (VSH). Researchers believe VSH behavior is probably triggered by the odors that are given off by the pupae that have been fed upon by mites and are infected with high levels of virus.
Some mites are infertile and these mites do not feed on pupae. Bees seldom open brood cells with mites that do not lay eggs. The USDA Baton Rouge Bee Lab has selected for this trait and made VSH bees available to queen producers. The Baton Rouge Bee Lab has also imported bees from Russia that have developed some ability to limit mite populations. It is not clear how the Russian bees do this but it is at least partly a result of the bees’ behavior.
“Selecting for Varroa Sensitive Hygiene” from eXtension describes methods for assessing VSH behavior in honey bees, articles.extension.org/pages/30984/selecting-for-varroa-sensitive-hygiene.The other trait that helps bees get rid of mites is grooming behavior. Some bee stocks are better at removing mites from their bodies than others. This behavior is also linked to mite-biting. Colonies that groom more mites from themselves tend to have a higher proportion of chewed mites on the sticky board sampling sheets.
Grooming behavior may interfere with monitoring mite levels with a sticky board because the bees are actively removing mites from themselves, but in general, most mites fall passively as adult bees emerge from brood cells or bees rub against each other in the hive, which dislodges mites. You can determine if mites from sticky boards have been chewed by putting them on their backs and examining them at 15 times magnification. This allows you to see the legs, which are more susceptible to chewing damage. A high proportion of chewed mites indicates relatively high grooming activity in the hive.
The Purdue University bee lab has conducted a breeding program to increase the level of mite grooming in bees, and some queens from these stocks are available from queen producers in the Midwest. Other sources of mite-grooming bees are being identified as queen producers become aware of the value of this trait.
When deciding where to purchase new queens, try to find sources that advertise mite tolerance or are produced in your region. Using local queens usually means that you must wait until June to purchase bees. However, remember that even mite-tolerant bee stocks can eventually collapse from heavy varroa mite infestations, so it is important to continue monitoring mite populations and use miticide when needed. You should also remember that when more susceptible colonies collapse from mite infestations, the mites from those colonies will end up in your other nearby hives. By keeping an eye on mite levels and using mite-tolerant stock, it is possible to keep winter hive losses to about 15 percent or less.
All photos were provided by and are the property of the authors except the cover photo by Tom Campbell, Purdue Agricultural Communication; and Figure 2 by Harry Laidlaw.
Reference in this publication to any specific commercial product, process, or service, or the use of any trade, firm, or corporation name is for general informational purposes only and does not constitute an endorsement, recommendation, or certification of any kind of Purdue Extension. Individuals using such products assume responsibility for their use in accordance with current directions of the manufacturer.
This publication is supported by the Office of Indiana State Chemist Pollinator Protection Plan. More information about the plan is available on the State Chemists' website: www.oisc.purdue.edu/pesticide/p3_activities.html.
This publication was partially funded by a Purdue Extension Issue-Based Action Team (IBAT) award.
April 2017

It is the policy of the Purdue University Cooperative Extension Service that all persons have equal opportunity and access to its educational programs, services, activities, and facilities without regard to race, religion, color, sex, age, national origin or ancestry, marital status, parental status, sexual orientation, disability or status as a veteran. Purdue University is an Affirmative Action institution. This material may be available in alternative formats.
This work is supported in part by Extension Implementation Grant 2021-70006-35390 / IND90001518G-1027053 from the USDA National Institute of Food and Agriculture.
765-494-8491
www.extension.purdue.edu
Order or download materials from edustore.purdue.edu