Pest & Crop Newsletter, Entomology Extension, Purdue University
- The Other Cutworms
- Slugs and Seed Slots
- Thanks to the Black Cutworm Pheromone Trap Cooperators
- Black Cutworm Adult Pheromone Trap Report
- Black Light Trap Catch Report
The Other Cutworms - (Christian Krupke, John Obermeyer, and Larry Bledsoe)
- There are many species of cutworms that feed on corn.
- Black cutworm is the most common and damaging species.
- The dingy cutworm is a “climbing cutworm” and rarely cuts plants.
- The dingy and clay-backed cutworm overwinter as partially grown larvae.
- Herculex 1 or XTRA may not control these other cutworms.
Many cutworm species look alike and identification is often confusing. Proper identification is critical because the black cutworm can be an economic threat to corn, whereas other species are typically not. As the name suggests, the black cutworm will cut or burrow into plant stems, causing stand losses. The black cutworm is our most commonly found species, but some fields will have a mixed bag of dingy, clay-backed, and variegated cutworms.
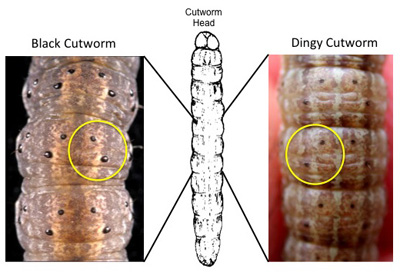
Dorsal surface of black (left) and dingy cutworm (right). Note tubercle sizes.
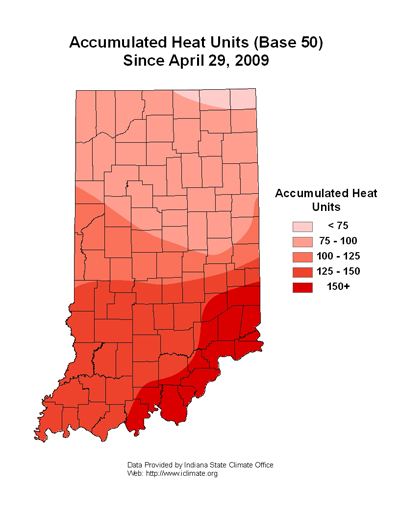
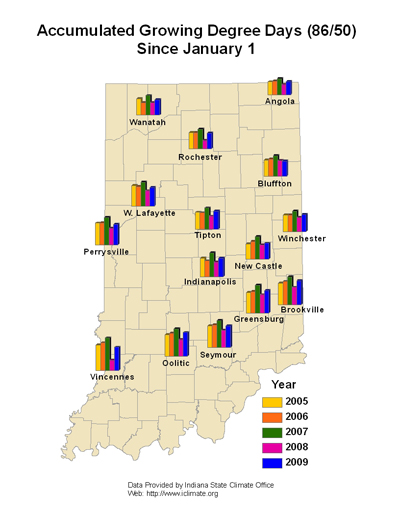
Black cutworms do not overwinter in the Midwest, which is why we monitor their arrival each spring with pheromone traps. Once they arrive in large numbers (i.e. intensive captures) we begin predicting their development and subsequent damage with heat unit accumulations (≥300 HU base 50ºF), see enclosed map. Fortunately, it has been cold as well as rainy this spring, and there have not been enough heat units accumulated this spring for black cutworms to get 1/2 to 3/4 inches long – the size when they begin to cut plants.
The dingy cutworm, probably the second most common species, is primarily a leaf feeder and will rarely cut plants, and if it does, the cutting is above ground level. Because a corn plant up to the 5-leaf stage can withstand severe defoliation without a yield loss (compare it to frost damage), treatment for the dingy cutworm is rarely justified. To confuse the issue even more, there are many other species that one may find while scouting. For example, the clay-backed cutworm is not as common as the black and dingy, and its damage is a mix of leaf feeding and plant cutting. The dingy and clay-backed cutworms overwinter as partially grown larvae, therefore finding cutworms 3/4 of an inch or more at this time would likely point to these species.
We received a call from southern Indiana last week concerning fair-sized cutworms damaging a field of Herculex corn. Two factors, the size of cutworm (lack of heat units) and poor control with Herculex led us to the fact that this was not black cutworm. Though physiologically, the cutworm species are similar, overwintering as larvae allows the dingy and clay-backed to be larger at the time of seedling (Bt) consumption, greatly reducing efficacy. No matter what toxin (Bt in the plant, sprays, granular insecticide), a larger insect is always more difficult to kill than a smaller one.
Below are some morphological characteristics that may help differentiate the black and dingy cutworm species.
Identification Features:
• do not use color!
• skin textures are different with 10X magnification (black = granular, dingy = smooth)
• tubercle (black warts or bumps) size on the top center of the body segments are different: the black’s outside pair are about twice the size of the inside pair, the dingy’s are all about the same size. Refer to the accompanying diagram.
![]()
Slugs and Seed Slots - (Christian Krupke, John Obermeyer, and Larry Bledsoe)
- Slugs are favored by a wet spring with heavy crop residue on the soil surface.
- Crop damage and stand losses are most severe when slugs enter open seed slots.
- Control options are limited.
With planting going at a “snail’s pace” recently, there will be a need to plant fields before soils are in their optimal condition. Over the last decade or so, slug populations have increased in various areas of the state, especially where heavy residue is left on the soil surface. Fields that have a history of slug problems with small grain or grass-type residues are especially vulnerable.
Most damage and stand losses by slugs occur when fields are planted wet and seed slots are not properly closed. In this situation, slugs can be found feeding on the seedlings within the slot, day or night. Control of slugs is difficult, if not impossible at this point. Soil insecticides applied during or post-planting and seed-applied insecticides are ineffective against slugs, as they slime over them. Bt corn has no effect on slugs. Once the growing point of corn or soybean is injured, plant recovery is unlikely.
An "open invitation for slugs!
Where slugs have been a problem in years past, tillage is a possible management strategy. Disrupting the soil environment exposes slug eggs and juveniles, destroying many and/or discouraging population growth. Although this not an option on highly erodible land (HEL), row cleaners used at planting may help. There are metaldehyde-pelleted baits that are labeled and available for use. Spreading the mini-pellets evenly over damaged areas is a challenge; though a commercial mechanical dispenser is one possibility. With the significant cost and difficulty of application, consider these baits only as a last resort to protect crop stands in high slug population areas.
![]()
Thanks to the Black Cutworm Pheromone Trap Cooperators! – (John Obermeyer)
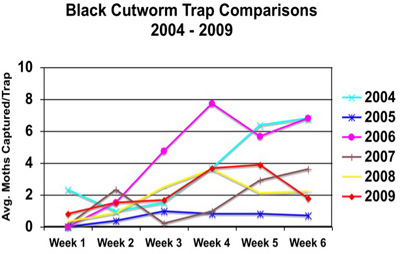
This is the last week of the “Black Cutworm Adult Pheromone Trap Report” for this year. As mentioned last week, this year’s moth captures haven’t been too impressive, and this past week hasn’t been any different, see graph of trap comparisons to past years. This doesn’t take away from the dedication these volunteers have shown in daily checking messy traps, plucking out captured moths, and reporting counts for the benefit of all of Indiana. Please look over the list of cooperators, should you know them, please thank them. It really is valuable information.
Now we wait for heat units to accumulate for the black cutworm development and corn to be planted and emerge. Soon it’s everybody’s favorite time…scouting!
![]()
Click here to view the Black Cutworm Adult Pheromone Trap Report
![]()
Click here to view the Black Light Trap Catch Report
In This POST Herbicide Era – Consider Using a Soil Applied Herbicide – (Tom Jordan, Bill Johnson, Glenn Nice, and Tom Bauman)
With trait resistant crops dominating the fields of the state, the majority of the herbicides applied for weed control are postemergence products. These have proven to be effective, economical, and easily worked into a spray schedule. There are however, several areas of the state where the level of weed control is not what it used to be, and indeed many weeds are becoming tolerant or resistant to the products that match the tolerant traits of the crops. These herbicide tolerant traits are in both corn and soybeans, allowing continuous use of the same mode of action yearly. As weed control performance decreases with these chemistries, consideration should be given to changing to herbicides with different modes of action for that field. Mixing modes of action and returning to a combination of soil applied herbicides followed by postemergence herbicides will help keep the weed control performance high and slow the development of weed populations that are tolerant or resistant to a particular mode of action.
No one herbicide will consistently control all weeds in a given field. When developing an herbicide program, first determine if the expected weed control from the herbicide that has been used is still acceptable or if it is beginning to decline over time. If you are not getting the control that you once got, it is indeed time to change your program. However, if you are satisfied with the control and are using only the chemistry that the crop was developed for, it may only be a short time before you realize a decrease in weed control. In either case, mixing herbicide modes of action is a safe way to maintaining acceptable weed control at an economical cost. It is important to know your weeds. Choose herbicides or herbicide mixtures that best control the worst weed problems in a field, and then, for those that escape the treatment, a timely second application of an herbicide that is active on those weeds may be needed. Research at Purdue and other universities has shown that, in fields where a POST herbicide treatment is not working to expectation, applying a PRE and following with a POST application improves weed control (Table 1). These data show that with a preemergence program, the consistency of weed control improves as indicated in the control values from the range of control observed over years and sites.
As weeds escape a particular set of POST herbicides and become increasingly hard to control, PRE herbicides, selected for those weeds, will minimize the selection pressure of the herbicide resistant or tolerant weeds in that field. Even if some of the weeds are not totally killed by the PRE treatment, weed growth is reduced allowing the POST application to be made to smaller weeds, ensuring better coverage and increased control. While herbicide resistant crops have allowed for a wider window of application over the past decade or so, as weeds become more tolerant or resistant to these herbicides, it is important to change to different modes of action or add an herbicide with a different mode of action to the herbicide program to keep weeds in check and crop yields at their maximum.
| Table 1. Percent (%) Weed Control in No-till, Narrow-row RR Soybeans With and Without a Residual Herbicide | |||
| Control | Herbicide Treatments | ||
| 3.4 oz Canopy XL Pre Followed by 2 pt Roundup Post | 2 pt Roundup Burndown Followed by 2 pt Roundup Post | 1.5 pt Roundup E Post Followed By 1 pt Roundup L Post | |
| (%) | (%) | (%) | |
| Averagea | 99 | 91 | 93 |
| Rangeb | 91-100 | 30-100 | 69-100 |
aFrom: Dirks, et.al., 2000 Weed Science Journal |
|||
![]()
Flexstar GT Reduced Rate Supplemental Label – (Glenn Nice)
The Flexstar GT present label specifies rates of 3 pt/A to 4.5 pt/A for use in glyphosate tolerant soybean. This rate structure provides 0.2 to 0.4 lb ai fomesafen and 1 to 1.5 lb ae glyphosate. Keep in mind that these rates are dependent on where you are in Indiana. If you are South of 1-70 you can use up to 4.5 pt/A, but if you are North of I-70 you are restricted to a maximum use rate of 3.75 pt/A.
A new supplemental label allows the reduced rate of 2.375 pt/A of Flexstar GT on non-glyphosate resistant weeds. This rate provides 0.19 lb ai fomesafen (0.8 pt/A of Flexstar) and 0.78 lb ae glyphosate. The supplemental label specifically mentions morningglory, velvetleaf, and black nightshade. Applications at this rate should be made on 2-4 inch weeds.
Watch for more information in our Herbicide News section on our website:
<http://www.btny.purdue/weedscience/herbicide/index.html>.
Wheat Viruses Confirmed in Indiana – (Kiersten Wise and Gail Ruhl)
The Purdue Plant and Pest Diagnostic Lab (P&PDL) has confirmed wheat streak mosaic virus (WSMV), wheat spindle streak mosaic virus (WSSMV), and soil-borne wheat mosaic virus (SBWMV) in several wheat samples over the last week (Figure 1). These samples were submitted from locations in north-central, northeast, and southwest Indiana. Multiple viruses were confirmed in several of the samples. A previous Pest&Crop newsletter article discussed the symptoms, detection, and management of the soil-borne wheat viruses: <http://extension.entm.purdue.edu/pestcrop/2009/issue2
/index.html#lookout>.
Virus diseases of wheat are difficult to tell apart in the field and require lab testing for accurate diagnosis. At this point in the season, there are no effective management strategies for controlling soil-borne viral diseases of wheat. Fungicide applications will not have an impact on viral diseases. It is extremely important to know what viruses are present in a field, because varieties that are resistant to one virus may not be resistant to other viruses.
The P&PDL provides testing for the presence of WSSMV, WSMV, SBWMV and 5 strains of BYDV with a multiplexed PCR detection assay. For an accurate diagnosis it is important to dig and submit entire plants exhibiting symptoms (see submission information at <http://www.ppdl.purdue.edu>). A minimal virus testing fee of $25.00 is made possible through supplemental diagnostics grant funds provided by the National Plant Diagnostic Network <http://www.npdn.org/>.
Figure 1. Wheat leaves with wheat spindle streak mosaic virus and wheat soil-borne mosaic virus.
![]()
Root Development in Young Corn - (Bob Nielsen)
Successful emergence (fast & uniform) does not guarantee successful stand establishment in corn. The next crucial phase is the establishment of a vigorous nodal root system. Success is largely dependent on the initial development of nodal roots from roughly V2 (2 leaves with visible collars) to V6.
Corn is a grass and has a fibrous type root system, as compared to soybeans or alfalfa that have tap root systems. Stunting or restriction of the nodal root system during their initial development (e.g., from dry soil, wet soil, cold soil, insect damage, herbicide damage, sidewall compaction, tillage compaction) can easily stunt the entire plant’s development. In fact, when you are attempting to diagnose the cause of stunted corn early in the season, the first place to begin searching for the culprit is below ground.
To better understand rooting development and problems associated with root restrictions, it is important to recognize that root development in corn occurs in two phases. The first phase is the development of the seminal or seed root system. The second phase is the development of the nodal or crown root system.
Corny Trivia: Sometimes you may hear the seminal root system referred to as the primary root system and the nodal root system as the secondary root system. This classification was described by Cannon (1949) and certainly makes chronological sense but always confuses me from the standpoint of importance to the plant.
The Seminal (Seed) Root System
Seminal (seed) roots originate from the scutellar node located within the seed embryo and are composed of the radicle and lateral seminal roots. The radicle emerges first and elongates towards the tip end of a kernel. The lateral seminal roots emerge later from behind the coleoptile and elongate towards the dent end of a kernel.
Elongation of the stalk tissue begins between leaf stages V4 and V5. Elongation of the internode above the fifth node usually elevates the sixth node above ground. Subsequent elongation of higher-numbered stalk internodes will result in higher and higher placement of the remaining stalk nodes. Sets of nodal roots that form at above ground stalk nodes are commonly referred to as “brace” roots, but function identically to those nodal roots that form below ground. If surface soil conditions are favorable (moist and not excessively hot), brace roots will successfully penetrate the soil, proliferate, and effectively scavenge the upper soil layers for water and nutrients.
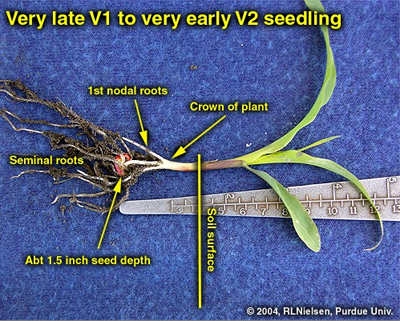
Seminal and nodal roots of V2 seedling
Corny Trivia: Root hairs are lateral extensions of root epidermal cells, grow to a length of several millimeters, and number about 200 per sq. millimeter (Gardner et. al., 1985). Their typical life span is only about 2 days at moderate temperatures and less so at higher temperatures (Gardner et al., 1985). Root hairs are visible even on the radicle root of a young seedling. Collectively, the surface area represented by root hairs is very large and can account for a large share of nutrient and moisture uptake by the plant.
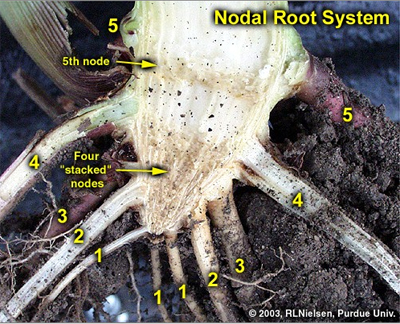
First five sets of nodal roots identified on a split stalk of corn.
A split stalk of an older plant will reveal a “woody” triangle of stalk tissue at the bottom of the corn stalk. This triangle is typically comprised of four stalk nodes, one on top of the other, whose associated internodes do not elongate. The first internode to elongate is the one above the fourth node, which elongates about 1/4 to 1/2 inch, above which is found the fifth node (usually still below or just at the soil surface). Consequently, five sets or whorls of nodal roots will usually be detectable below ground (one set for each of the below ground stalk nodes).
Effects of Root Damage in Young Corn Plants
Even though the seminal root system contributes little to the season-long maintenance of the corn plant, early damage to the radicle or lateral seminal roots can stunt initial seedling development and delay emergence. Such damage will not necessarily cause immediate death of the seedling as long as the kernel itself and mesocotyl remain healthy, but may result in the seedling leafing out underground. As more and more nodal roots become established over time, damage to the seminal root system will have less and less impact on seedling survival.
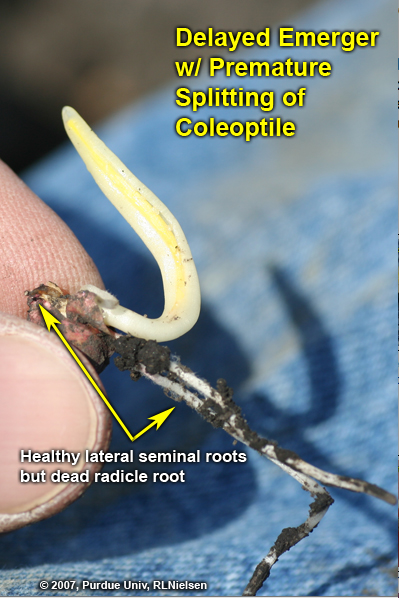
Examples of seminal root damage include imbibitional chilling injury, post-germination injury from lethal or sub-lethal cold temperatures, and “salt” injury from excessive rates of starter fertilizer placed too close to the kernel. Symptoms of such root damage include retarded root elongation, brown tissue discoloration, prolific root branching, and outright death of root tissue. If the radicle root is damaged severely during its emergence from the kernel, the entire radicle root may die. Once the radicle has elongated a half-inch or so, damage to the root tip will not necessarily kill the entire root, but rather axillary root meristems may initiate extensive root branching in response to damage to the apical meristem.
Delayed emerger with healthy lateral seminal roots but damaged radicle root. Coleoptile is splitting prematurely and seedling will likely leaf out underground.
Corn seedlings transition from dependence on kernel reserves to dependence on nutritional support by the nodal roots around the V3 leaf stage. Damage or stress to the first few sets of developing nodal roots during the time period V1 to V5 can severely stunt or delay a corn plant’s development. Damage to the first few sets of nodal roots forces the young seedling to continue its dependence on kernel reserves longer than is optimum. If the kernel reserves are nearly exhausted, continued seedling development is easily stunted and seedling death is not uncommon. Typical stresses that can stunt initial nodal development include fertilizer salt injury, seedling diseases, herbicide injury, insect feeding damage, excessively wet or dry soils, soil compaction (tillage or planter).
Corny Trivia: The primary meristem of a root is located near the root tip. Elongation of cells behind the meristem leads to elongation of the root.
A somewhat uncommon, but dramatic, stunted root symptom is what is referred to as the “floppy corn” or “rootless corn” phenomenon. This problem occurs as a result of the detrimental effects of excessively dry surface soil near the time of initial nodal root elongation in young (V2 to V4) corn plants. Young nodal roots that emerge from the crown area of the plant will die if their root tips (and associated meristematic areas) desiccate prior to successful root establishment in moist soil. The crown of a young corn plant is typically located only 3/4 inch or so below the soil surface and so is particularly vulnerable to dry upper soil conditions.
Related References
Cannon, William Austin. 1949. A Tentative Classification of Root Systems. Ecology 30[4], 542-548.
Gardner, Franklin P., R. Brent Pearce, and Roger L. Mitchell. 1985. Physiology of Crop Plants. Iowa State Univ. Press, Ames, IA.
Nielsen, RL (Bob). 2004. Over-Extended Mesocotyls and Floppy Corn Syndrome. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/articles.04
/FloppyCorn-0624.html>. (URL accessed 5/5/09).
Nielsen, RL (Bob). 2007. Be on the Alert for Floppy, Rootless Corn. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/articles.07/Floppy-0523.html>. (URL accessed 5/5/09).
Nielsen, RL (Bob). 2009a. Germination Events in Corn. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/timeless/GerminationEvents.html>. (URL accessed 5/5/09).
Nielsen, RL (Bob). 2009b. The Emergence Process in Corn. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/timeless/Emergence.html>. (URL accessed 5/5/09).
Nielsen, RL (Bob). 2009c. Visible Indicators of Germination in Corn. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/timeless/
GerminationGallery.html>. (URL accessed 5/5/09.
![]()
Einstein’s Theory of Relativity as it Applies to Soil Moisture - (Bob Nielsen)
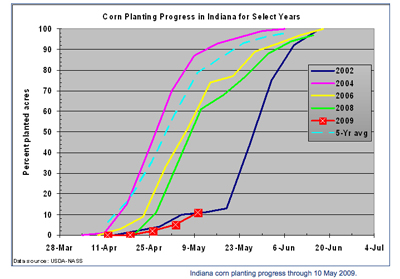
The good news is that Monday’s USDA-NASS report showed that Indiana’s corn planting progress had caught up to that of the same time period in 2002. The bad news is that the 2002 planting season was one of the slowest in recent history. With a forecast of more rain throughout the state the middle of this week, let me offer a contrarian view about soil moisture and planting.
The superintendent of our Purdue Agronomy Farm and I share a laugh every planting season when it comes to deciding when the soil is “fit” to work or plant. We scuff around the fields in mid-April, dig a few spadefuls of soil, squeeze the soil into a ball like the soil scientists tell us to do, and then agree that the soil is too wet to work or plant. Around the first of May, we scuff around the fields, dig a few spadefuls of soil, squeeze the soil into a ball like the soil scientists tell us to do, and then agree that the soil is too wet to work or plant. Again in mid-May, we scuff around the fields, dig a few spadefuls of soil, squeeze the soil into a ball like the soil scientists tell us to do, and then agree that the soil is maybe just about right to work or plant, but we’ll give it a few more days. By late May, we scuff around the fields, dig a few spadefuls of soil, squeeze the soil into a ball like the soil scientists tell us to do, and then agree that the soil is just as wet as it was back in mid-April, but maybe we ought to be working ground and planting anyway. Einstein was right............it’s all about relativity.
The point of my sharing this annual ritual with you is that we are rapidly approaching the point in the planting season where we need to “fish or cut bait.” Yes, there are risks of working ground too wet or planting “on the wet side” (see articles below), but there are also risks of waiting so long for the soil to become “fit” to begin planting that the majority of your corn ground gets planted way too late.
Heaven forbid that I should recommend anyone to work ground or plant corn in soils that are wet enough to cause severe compaction that will haunt you later this summer. But, you know, when you decide back in mid-April to wait, you’ve got quite a bit of good planting season left to go. When you decide in mid-May to wait AND you have a lot of acres to cover, what you save by avoiding some soil compaction now may be less than what you risk by planting the bulk of your corn acres very, very late.
If you concur with these thoughts, “mud in” your corn, and suffer serious yield losses; you did not hear it from me. If you “pull the trigger” now and successfully avoid planting the bulk of your corn in mid-June and win the yield jackpot; then I’ll accept all the credit.
Tractor stuck in a muddy field
There are no black & white answers to this situation, there are no silver bullets, and there are no certainties in farming. Use your best judgement in deciding when to head back to the fields over the coming days and/or weeks. You know your fields and soils better than anyone else.
Related References
Duiker, Sjoerd. 2009. Planting into wet soil? Field Crop News, Pennsylvania State Univ. [online] Available at <http://fcn.agronomy.psu.edu/2009/fcn0907.cfm#h>. [URL accessed 5/11/09]
Graybill, Jeff. 2009. Should I Be “Mudding in” My Corn?. Field Crop News, Pennsylvania State Univ. [online] Available at <http://fcn.agronomy.psu.edu/2009/fcn0909.cfm#g>. [URL accessed 5/11/09]
Murdock, Lloyd. 2009. Avoiding Sidewall Compacting During Late Corn Planting. Corn and Soybean Newsletter. Univ of Kentucky. [online] Available at <http://www.uky.edu/Ag/CornSoy
/cornsoy9_4.htm#2>. [URL accessed 5/11/09]
Thelen, Kurt. 2009. Exercise patience in deciding when to commence field operations. Field Crop Advisory Team Alert. Michigan State Univ. [online] Available at <http://www.ipmnews.msu.edu/fieldcrop/fieldcrop/tabid/56/articleType/ArticleView
/articleId/176/Exercise-patience-in-deciding-when-to-commence-field-operations.aspx>. [URL accessed 5/11/09]