Pest & Crop Newsletter, Entomology Extension, Purdue University
- Alfalfa Weevil Development Continue Northward, Management Problematic
- No Black Cutworms Yet...Very Little Corn Either
- Nematode Updates
- Black Light Trap Catch Report
- Black Cutworm Adult Pheromone Trap Report
Alfalfa Weevil Development Continues Northward, Management Problematic – (Christian Krupke and Larry Bledsoe)
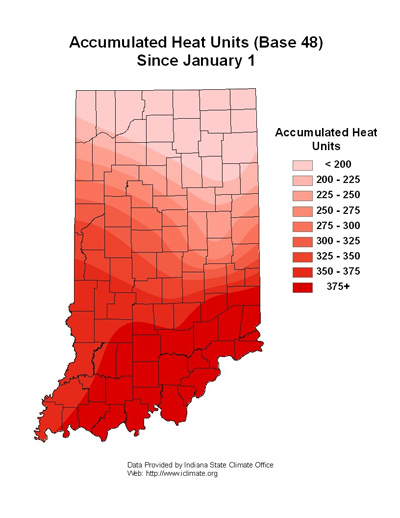
Warmer conditions have accelerated alfalfa weevil development in the northern Indiana Counties, see accompanying map, “Accumulated Heat Units (Base 48).” Scouting for weevil damage is encouraged at 200 or more heat units. As previously mentioned, alfalfa cutting and/or weevil management has been a challenge this soggy spring. Refer to last week’s management guidelines and recommended insecticides.
Brownish weevil, in the middle, is diseased and dying.
Silver lining within our dark clouds: David Trotter, Clark County CES, inspected some fields this week and found discolored (yellowish to brown) alfalfa weevil larvae. It is likely that these larvae are infested with the insect fungal pathogen. Zoophthora phytonomi. One of many fungi that attack and kill insects, this disease has occurred because of the abundance of moisture we have received. Once this disease is established in a field with high humidities, weevil larvae can quickly diminish. Unfortunately, weevil populations are usually pretty high before this disease is effective at controlling them, so we cannot rely upon it for control.
![]()
No Black Cutworms Yet…Very Little Corn Either – (Christian Krupke and John Obermeyer)
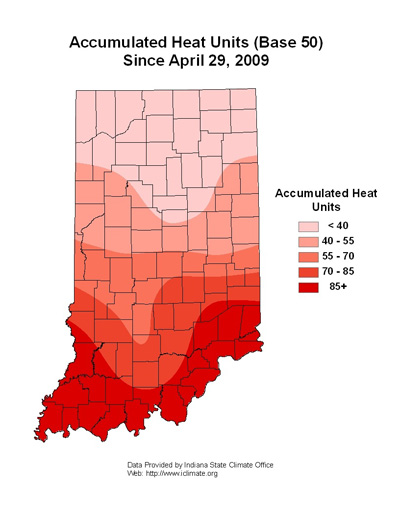
Two intensive black cutworm moth captures, 9 or more caught over a 2-night period, last week signaled for us to begin tracking heat units (Base 50), see following map. Based on the black cutworm’s growth development model, it takes approximately 300 heat units (base 50ºF) from egg hatch to early 4th instar; this is when black cutworm larvae begin to cut plants. Some leaf injury may be present before then.
In referring to the map, it is too early for black cutworm scouting. Unfortunately, only about 5% of the corn is planted, but weedy fields for egg laying are abundant. Consider the timing of corn emergence, yet to be planted, and the progression of cutworm development in the next week or two. The good news is that, except for a few very active pheromone traps, overall moth captures have been lower than typical. Stay tuned.
![]()
Nematode Updates - (Jamal Faghihi, Christian Krupke, and Virginia Ferris)
Most of you have already decided what soybean cultivars you are going to plant. If you have had problems with SCN in the past, you probably have chosen one or more of the “cyst-bean” cultivars to plant. You might need to pay closer attention to the source of resistance for these cultivars. As we indicated previously, field populations of SCN in Indiana are changing in ways that render less effective the most common source of resistance to SCN (PI 88788). Other researchers in the region have reported similar trends. Our collaborative NCRSRP-funded research indicated that Peking and PI437654 sources of resistance are still very effective sources of resistance to SCN in Indiana and you might want to consider these when choosing soybean cultivars.
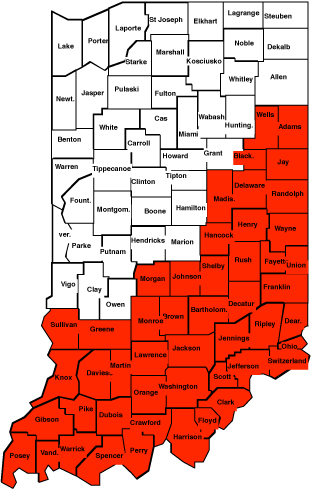
To have a better understanding of the SCN distribution in Indiana, we travelled throughout Indiana in 2008 and collected many samples (supported by a one-year grant from ISA). However, we need additional samples from counties that are colored in red. We would like to encourage growers who farm in these counties to send us at least one sample from their farm. The customary processing charge of $10/sample will apply but any additional work that we do with these samples will not be charged to you. We will expose these samples to the most common source of resistance, PI88788. If these samples qualify for HG type testing we will perform the test and will send you a copy of the result with no extra cost to you. You can sample for SCN anytime of the year regardless of the current or previous crops. Soil samples taken from a depth of 4-6 inches can be sent to: Nematology laboratory, Purdue University, Department of Entomology, Smith Hall, 901 W. State Street, West Lafayette, IN 47907-2089.
One might ask why further soil testing is needed if SCN is already known to be present. The answer to this question is that soil tests are the only way you can find out whether your SCN population is changing and whether your resistant variety is still working. All growers need to understand that changes in SCN field populations are gradual and one should not wait for drastic yield losses before finding out that a change in the SCN population has occurred. Growers can look for white and yellow SCN females on the roots about two months after planting. Or they can send soil plus root samples in the middle of the growing season to our nematology laboratory to determine the effectiveness of resistant varieties growing in the field. If race determination has been performed for a field population in the past, you might want to repeat the test after about four years of growing soybeans to measure any possible changes. If no profile exists, one must be established so future comparisons are possible. In short, planting SCN resistant varieties is not the final solution to the soybean cyst nematode problem. The management of this pest is an ongoing and dynamic operation requiring constant vigilance.
The best way to manage SCN over the years is to monitor your populations by sampling each field at least every four years. You can sample the soil anytime of the year and get an accurate understanding of the cyst population. This is a very crucial step in SCN management and should not be neglected. We provide this service to soybean growers at the cost of $10/sample, for which the submitter will receive a bill unless we are instructed otherwise. For more information on other services that we provide you may visit the Nematology website: <http://www.entm.purdue.edu/nematology/>
If you have any questions about SCN or any other kinds of plant parasitic nematodes, you can contact Jamal Faghihi at 765-494-5901 or send an email to <jamal@purdue.edu>.
![]()
Click here to view the Black Cutworm Adult Pheromone Trap Report
![]()
Click here to view the Black Light Trap Catch Report
![]()
Fusarium Head Blight Update – (Kiersten Wise)
Wheat in far southern Indiana reached flowering (10.5.1) this week, and wheat throughout southern Indiana is in late boot stage or beginning to head. Wheat in central to northern Indiana is still variable, ranging from early boot to just past jointing (Feekes 7). In southern Indiana this is an important time to consider the risk for Fusarium head blight development in wheat.
Last week, we discussed the Fusarium head blight prediction model and disease management in the Pest&Crop Newsletter <http://extension.entm.purdue.edu/pestcrop/2009/issue5/index.html#wheat>. To summarize what we discussed last week, Fusarium head blight development depends largely on weather conditions, primarily rainfall, humidity, and temperature. High humidity (80%), dew formation, frequent rainfall, and temperatures in the high 60s to mid 80s°(F) favor disease development. Weather conditions have been favorable for Fusarium head blight development in far southern Indiana, and growers should be checking the model to determine the approximate level of risk for their location <http://www.wheatscab.psu.edu/>. Additionally, it is important to scout fields in southern Indiana to determine the exact growth stage of wheat for optimum timing of any potential fungicide applications.
A few points to remember if fungicide applications are considered:
Fungicides should be applied at Feekes 10.5.1 for optimum control of Fusarium head blight. There are several fungicides available for Fusarium head blight control. These products are listed in the fungicide table that we discussed last week <http://www.ppdl.purdue.edu/ppdl/wise/NCA_184wheatfungicides.pdf>. Caramba, Prosaro, and Proline were given a rating of “good” based on a designation system from the Regional Wheat Disease Committee. Products containing only tebuconazole (Folicur, others) were rated as fair, and propiconazole alone (Tilt, others ) was rated as poor for management of Fusarium head blight. Remember, DO NOT apply fungicides that have a strobilurin mode of action (Headline, TwinLine, Quadris, Quilt, Stratego) for Fusarium head blight control.
![]()
Germination Events in Corn - (Bob Nielsen)
Germination is the renewal of enzymatic activity that results in cell division and elongation and, ultimately, embryo emergence through the seed coat. Germination is triggered by absorption of water through the seed coat. Corn kernels must absorb (imbibe) about 30 % of their weight in water before germination begins. Less than optimum absorption of water (perhaps due to a rapidly drying seed zone) may slow or stop germination. Repeated wetting/drying cycles can decrease seed viability.
By comparison, soybeans must imbibe about 50 % of their weight in water. But since soybeans are approximately 2/3 the weight of corn kernels, the total amount of absorbed water required for germination is relatively similar.
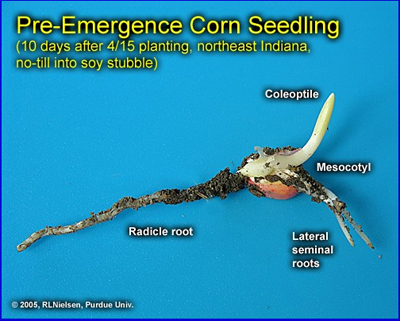
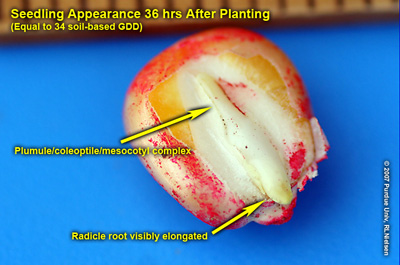
The visual indicators of germination occur in a distinct sequence. The radicle root emerges first, near the tip end of the kernel, within two to three days in warm soils with adequate moisture. In cooler or drier soils, the radicle root may not emerge until one to two weeks after planting.
The coleoptile (commonly called the “spike”) emerges next from the embryo side of the kernel within one to many days of the appearance of the radicle, depending on soil temperature. The coleoptile initially negotiates its way toward the dent end of the kernel by virtue of the elongation of the mesocotyl. The coleoptile is a rigid piece of plant tissue that completely encloses the four to five embryonic leaves (plumule) that formed during grain development of the seed production year. The plumule leaves slowly enlarge and eventually cause the coleoptile to split open as it nears the soil surface.
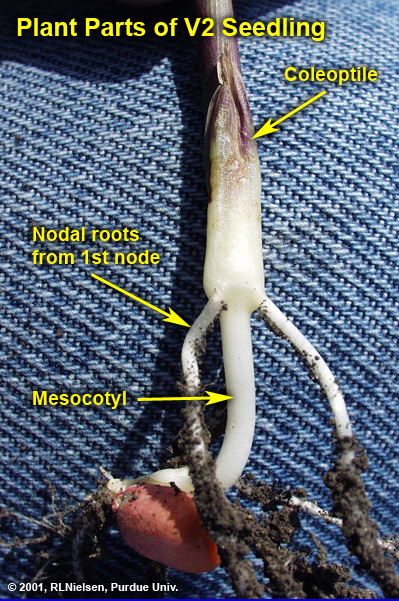
The lateral seminal roots emerge last, near the dent end of the kernel. Even though these and the radicle root are technically nodal roots, they do not comprise what is typically referred to as the permanent nodal root system. The first set of so-called “permanent” roots begins elongating at approximately the V1 leaf stage (1 leaf with visible leaf collar) and is clearly visible by V2.
Click here to see more of the Visible Indicators of Germination in Corn <http://www.agry.purdue.edu/ext/corn/news/timeless/GerminationGallery.html>.
Troubleshooting Considerations
When temperatures are optimum, these three parts of the seedling may emerge from the kernel on nearly the same day. Excessively cool soils may delay the appearance of the coleoptile and lateral seminal roots for more than a week after the radicle root emerges. It is not uncommon in cold planting seasons to dig up kernels two weeks after planting and find only short radicle roots and no visible coleoptiles.
When excessively cold and/or wet soils delay germination and/or emergence, the kernel and young seedling are subjected to lengthier exposure to damaging factors such as soil-borne seed diseases, insect feeding and injury from pre-plant or pre-emergent herbicides and carryover herbicides from a previous crop.
Related References
Hick, Dale and Seth Naeve. Date unknown. The Corn Growers Field Guide For Evaluating Crop Damage And Replant Options. Univ. of Minnesota Ext. Service. [On-Line]. Available at <http://www.soybeans.umn.edu/pdfs/CornGuide.pdf>. (URL accessed 5/5/09).
Nielsen, RL (Bob). 2009a. Requirements for Uniform Germination and Emergence of Corn. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/timeless/GermEmergReq.html>. (URL accessed 4/28/09).
Nielsen, RL (Bob). 2009b. The Emergence Process in Corn. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/timeless/Emergence.html>. (URL accessed 4/28/09).
Ritchie, S.W., J.J. Hanway, and G.O. Benson. 1993. How a Corn Plant Develops (SP-48). Iowa State Univ. [On-Line]. Available at <http://www.extension.iastate.edu/hancock/info/corn.htm>. (URL accessed 5/7/08).
![]()
The Emergence Process in Corn - (Bob Nielsen)
Successful germination alone does not guarantee successful emergence of a corn crop. The coleoptile must reach the soil surface before its internal leaves emerge from the protective tissue of the coleoptile. Growth stage VE refers to emergence of the coleoptile or first leaves through the soil surface (Ritchie et. al., 1993).
As with all of corn growth and development, germination and emergence are dependent on temperature, especially soil temperature. Corn typically requires from 100 to 120 GDD (growing degree days) to emerge. Under warm soil conditions, the calendar time from planting to emergence can be as little as 5 to 7 days. Under cold soil conditions, emergence can easily take up to four weeks.
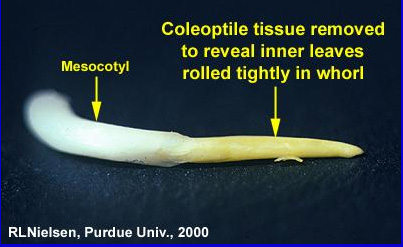
Elongation of the mesocotyl elevates the coleoptile towards the soil surface. The mesocotyl is the tubular, white, stemlike tissue connecting the seed and the base of the coleoptile. Technically, the mesocotyl is the first internode of the stem.
Useful Tip: Physiologically, mesocotyls have the capability to lengthen from at least a 6-inch planting depth. Realistically, corn can be planted at least three inches deep if necessary to reach adequate moisture.
As the coleoptile nears the soil surface, exposure of the mesocotyl to the red light portion of the solar radiation spectrum halts mesocotyl elongation. Continued expansion of the leaves inside the coleoptile ruptures the coleoptile tip, allowing the first true leaf to emerge above the soil surface. Since the depth at which the mesocotyl senses red light is fairly constant, the resulting depth of the crown (base) of the coleoptile is nearly the same (1/2 to 3/4 inch) at seeding depths of one-inch or greater.
Useful Tip: When corn is seeded very shallow (less than about 1/2 inch), the crown of the coleoptile will naturally be closer to the soil surface if not right at the surface. Subsequent development of the nodal root system can be restricted by exposure to high temperatures and dry surface soils.
Click here to see more Coleoptile appearance during emergence <http://www.agry.purdue.edu/ext/corn/news/timeless/ColeoptileGallery.html>.
Troubleshooting Considerations
Several factors can cause the coleoptile to split prematurely, allowing the leaves to emerge underground. Usually, more than one of the following factors are present when this problem occurs, making it difficult to place the blame on any one factor.
Exposure to light at deeper soil depths than usual due to cloddy seedbeds, dry seedbeds, sandy soils, or open slots in no-till.
Injury from certain herbicides, particularly under stressful environmental conditions. Symptoms include corkscrewed coleoptile, swollen mesocotyl and true leaves emerged from side of coleoptile.
Surface crusting, cloddy seedbeds, rocky seedbeds, planter furrow compaction, or otherwise dense surface soil that physically restrict mesocotyl elongation and coleoptile penetration. The pressure of the expanding leaves within the coleoptile eventually ruptures the side of the coleoptile. Symptoms include corkscrewed coleoptile, swollen mesocotyl and true leaves emerged from side of coleoptile. Note the similarity to those symptoms from herbicide injury.
Cold temperature injury, either from exposure to long periods of soil temperatures around 50°F or from exposure to wide daily swings (25 to 30°F) in soil temperatures. Symptoms include absence of emerged coleoptile, corkscrewed mesocotyl or coleoptile and true leaves emerged from side of coleoptile. Note the similarity to those symptoms from herbicide injury.
Useful Tip: The mesocotyl should remain firm, white and healthy through at least the 6-leaf stage, if not longer. If it is mushy, discolored, or damaged prior to this stage, then it is likely part of the crop problem being investigated.
Related References
Nielsen, RL (Bob). 2008. Heat Unit Concepts Related to Corn Development. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/timeless/HeatUnits.html>. (URL accessed 5/5/09).
Nielsen, RL (Bob). 2009a. Germination Events in Corn. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/timeless/GerminationEvents.html>. (URL accessed 4/28/09).
Nielsen, RL (Bob). 2009b. Corkscrewed Corn Seedlings. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/articles/timeless/Cornscrews.html>. (URL accessed 4/28/09).
Nielsen, RL (Bob). 2009c. Requirements for Uniform Germination and Emergence of Corn. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/timeless/GermEmergReq.html>. (URL accessed 4/28/09).
Ritchie, S.W., J.J. Hanway, and G.O. Benson. 1993. How a Corn Plant Develops (SP-48). Iowa State Univ. [On-Line]. Available at <http://www.extension.iastate.edu/hancock/info/corn.htm>. (URL accessed 5/5/09).