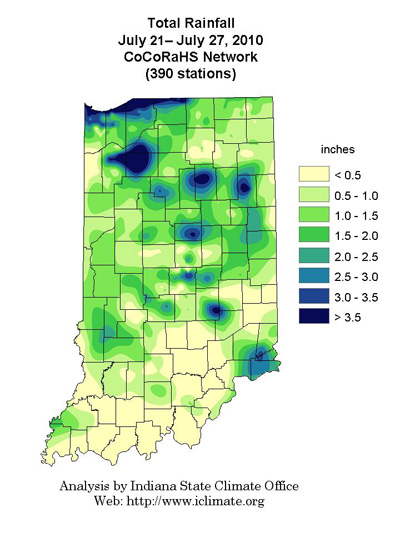
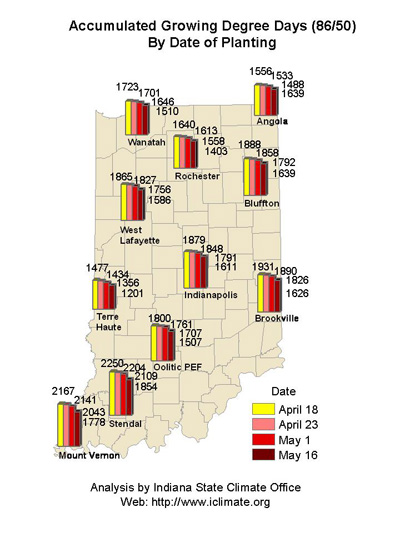
Pest & Crop Newsletter, Entomology Extension, Purdue University
- Western Bean Cutworm Damage a Surprise in Some Northern Indiana Fields
- Soybean Aphid Update
- Little Corn Borer Like Moths Flying at Night
- Black Light Trap Catch Report
- Western Corn Cutworm Adult Pheromone Trap Report
Western Bean Cutworm Damage a Surprise in Some Northern Indiana Fields – (Christian Krupke and John Obermeyer)
- Moth flight and egg laying is nearly complete, now it’s caterpillar time.
- Larval damage seems most severe in sandier areas of NW counties.
- By now, most larvae have entered the ear, presenting control challenges.
- Consider the factors listed below before attempting treatments.
Like last year, several pest managers in northern Indiana counties have been tracking this pest throughout the moth flight and egg laying period, and now are scouting for the larvae. Many are frustrated because egg masses found were well below the 5% plants infested threshold, but are now obviously infested with larvae. Several compounding factors are likely creating this “surprise” infestation. First, although there is a definite peak in trap catch, western bean cutworm moth flight occurs over several weeks (4-6 or so). For some fields, this adds up to a constant barrage of new eggs. During that period, egg mass scouting must occur at least weekly, shorter intervals being better. Female moths are picky on which plants they lay their eggs (e.g., color, growth stage, architecture, etc.) resulting in a clumped distribution in a field. This will be even more apparent in the fall, when very patchy damage is evident. Visiting multiple locations throughout a field increases the chance of finding the concentration of infestation. Consider that each egg mass may produce 20-50 larvae. Even with 70-80% larval mortality from abiotic and biotic factors, the survivors will spread out to neighboring plants. In other words, 1 egg mass equals multiple larvae…insect population dynamics!
Gently pulling back these silks revealed WBC frass, with the larva found at the ear tip
Small WBC larva and damage revealed after pulling the shucks back beyond the ear tip
The sizes of WBC larvae likely found in ears at this time
The current challenge is to identify fields that are infested, assess the size and location of the larvae, and determine if treatments are warranted. In at least ten different areas of the field, carefully examine the ear and ear zone of 10 consecutive plants. Include the secondary ear in your examination. Determine the percentage of plants infested and the size and activity of the larvae. This will require peeling back the husk over the ear tip to look for a worm and/or frass and/or damage. Also carefully pull back leaves and leaf sheaths adjacent to the ear. Again you may find larvae, and entrance holes into the side of the ear. Smaller larvae, <1”, seem to be more active in and out of the ear. Larger larvae seem content to remain in the ear and feed on kernels. As temperatures increase, the larvae are more likely to remain inside the ear.
Treatment for field corn with the majority of larvae in the ear is iffy at best. Remember that our foliar sprays are all contact insecticides and a larva in the ear isn’t contacting any outside surfaces - which is where all the insecticide residue will be. We have received reports from folks that treated last week (July 19) and were pleased with the results (please follow the Restricted Entry Interval that is on the product’s label). Since that time, larvae have grown and temperatures remain warm. Consider the following before treating:
• Control in corn that has already pollinated, will likely be less than 50%.
• 1 larva/ear at dent stage corn is approximately equal to a 4 bushel/acre loss (Nebraska and Iowa data).
• Ear damage opens the door for molds, a concern in food grade corn.
• Larvae in the ear will NOT be controlled, larvae exposed or that exit the ear can be.
• Larvae become less mobile as temperatures increase.
• Increased carrier volume will improve the canopy penetration into the ear zone.
• Insecticides will provide about a week of efficacy, give or take a few days depending on the environment (e.g., rain, heat, sunshine).
• Pre-Harvest intervals for insecticides, on the label, must be followed (most are 21 to 30 days).
• Bt hybrids containing Cry1A (YieldGard®) do not suppress or control western bean cutworm, those containing Cry1F (Herculex®, SmartStax®) do.provide control.
• Approved insecticides, their rates, and pre-harvest intervals can be viewed at: <http://extension.entm.purdue.edu/publications/E-219.pdf>, look under western bean cutworm.
![]()
Soybean Aphid Update – (Christian Krupke and John Obermeyer)
It has been some time since giving a soybean aphid update, so being prompted by those calling and wondering…there is no new news! Thanks to those who have been out looking and reporting that soybean aphid numbers continue to be extremely low, almost to the point of non-existent in the state. Reports from “aphid central” (i.e., Minnesota and Wisconsin) are much the same, much lower than normal numbers. David Ragsdale, University of Minnesota Entomologist, reports that abundance of heavy rains has decreased aphid numbers, some mortality measured over 60% with single rain events.
As we venture into the critical pod–forming and filling stages of soybean, low aphid numbers is very good news for producers!
![]()
Little Corn Borer Like Moths Flying at Night – (John Obermeyer)
Observations at my night-lit kitchen window and reports from Mike Gray, University of Illinois Entomologist, and Jeff Phillips, Tippecanoe County ANR Extension Educator are of an abundance of small European corn borer-like moths. There are so many of these moths flying at night that Jeff thought he was getting windshield splatter from second generation corn borer. These moths have been identified as the celery leaftier (Udea ribigalis), which is not a pest of field crops.
The celery leaftier has many hosts such as flowers and weeds, other than the obvious … celery. These moths have several generations per season, so it is possible we will see it again late this summer or early fall. Protect that celery crop!
European corn borer (top) compared to celery leaf-tier (bottom) moths
![]()
Click here to view the Black Light Trap Catch Report
![]()
Click here to view the Western Bean Cutworm Adult Pheromone Trap Report
Scouringrush Encroaching on Agricultural Turf - What We Know So Far – (Glenn Nice, Tom Jordan, Bill Johnson, and Tom Bauman)
Scouringrushs and horsetails are known by many different common names: snake grass, jointed grass and monkey grass or simply Equisetum to name a few. They all belong to the genus Equisetum and the USDA’s plant database indicates that there are 13 species in the Mid-West. The species are separated into different species by stem thickness, frequency of the vegetative form, stem height and various other subtle clues. One thing that is unique about equisetums is that they do not produce flowers or seed. This is an old group of plants that produce spores. To learn more about its life cycle please read “The Ancient Horsetail (WS-29-W).” <http://www.btny.purdue.edu/weedscience/2003/Articles/Horsetail03.pdf>. For the purpose of this article, the two species that we typically deal with, field horsetail (Equisetum arvense) and scouringrush horsetail (E. hyemale), will be referred to as horsetail and scouringrush, respectively.
Typically not a problem in agriculture, Equisetum is more of a problem around ponds and in ditches; however, both horsetail (an Equisetum that produces a small branched vegetative stems) and scouringrush (a species that only produces the reproductive stems) often find their way into agricultural fields. Both species prefer wet environments for reproduction, but can expand by rhizomes into dryer environments. In Northern Indiana, scouringrush expands from drainage canals into production fields leading to the need for control. The Weed Science team receives a number of calls every summer concerning the control of these weeds.
The most common control used by growers having scouringrush or horsetail encroaching on their production fields is to use tillage on a regular basis. In a study conducted in Canada, sixteen hoeing events were reported to have no impact on regrowth the following season[1]. This suggests that a continued mowing program alone would not be effective.
The inability to control Equisetum with herbicides is reported, both in the literature as well as by word of mouth. The lack of surface area as well as the structure of the hollow and siliceous nature of the stems may all contribute to inhibit herbicide entry into the plant. The success of controlling the above ground plant relies on control of the underground portion of the plant. There has been work to find suitable herbicides with activity. Peter Sikkema of Guelph University, Canada, reported more than 80% control of field horsetail with combinations of glyphosate and flumetsulam[2]. Flumetsulam is the active ingredient of commercially available herbicide Python®. Work done in Michigan reported 77% to 92% control with Curtail M® (MCPA + clopyralid)[3].
Much of the above work has been done on horsetail. However, in Northern Indiana we often deal with scouringrush. This species does not show the vegetative stem structures found in horsetail, but only the reproductive stems. Much work is needed to provide a greater body of data regarding the control of Equisetuem.
A field trial was conducted that looked at various herbicides, both labeled for crop use and not labeled in crops. Products labeled in crops consisted of Python®, Hornet®, Roundup Weathermax®, Sharpen®, Gramoxone Inteon® and atrazine. Products investigated that are not labeled in row crops were Milestone®, Habitat®, and Element®. Non-crop products were included because of the realization that to adequately control scouringrush the infested area may need to be taken out of production. Herbicide treatments were applied on mowed and non-mowed plots (Figure 1). Treatments were applied in the spring (April) and in the fall (November) with variable results.
Figure 1. Applications made over the top of non-mowed scouringrush. Dense colonies of scouringrush is not easy to walk through.
Non-Mowed
Roundup Weathermax® had no observable impact on scouringrush. Some of the treatments induced a color response by turning the scouringrush black (Figure 2). This discoloration was most evident in the treatments that included Gramoxone Inteon®, atrazine, and Ignite 280®. However, it should be noted that atrazine can not be used within 66 feet of the canal itself. The use of Gramoxone Inteon® alone or with atrazine decreased biomass 24% and 31% at 99 days after treatment, respectively (Figure 3). None of the over-the-top treatments adequately reduced the biomass of scouringrush. Mowing was required to produce acceptable results.
Figure 2. Discoloration of scouringrush after Gramozone Inteon, Gramozone Inteon + atrazine, and Ignite 280 treatments.
Figure 3. Die back from Gramoxone Inteon applications. Stems turned black then died leaving a mat of dean material. New growth can be seen growing up out of the old growth.
Mowed
As would be expected, mowing reduced biomass but regrowth occurred. Stem counts were taken in the mowed plots during mid-summer after spring applications in 2009 and 2010. The 2009 stem counts were done on plots that had only a spring application. The counts in 2010 were taken in plots that had seen two years of spring applications or one fall application. In the 2009 summer counts, before the fall-applied treatments were applied, Milestone® had the lowest amount of regrowth at 4 stems per sq. ft. (Figure 4.) The greatest average regrowth with in the mowed plots at this counting was 32 stems per sq. ft (Figure 5). The Python® and Hornet® treatments did have intermediate suppression of regrowth with 20 and 19 stems per sq. ft. on average.
Figure 4. Regrowth suppression from the mowed Milestone spring treatments in 2009.
Figure 5. Maximum regrowth of scouringrush in mowed plot approximately 31 stems per sq. ft.
Figure 6. Habitat applied in the fall showing no regrowth. Picture take 200 days after fall applications.
In the following season, stem counts were taken on June 1, at 42 days after spring applications and 200 days after fall applications. At the time of stem counts there was little to no regrowth in plots that received Habitat® applications in the fall (Figure 6). Mowed plots that received treatments with Milestone, Gramoxone Inteon®, atrazine, and Sharpen® had stem counts between 8 and 19 stems per sq/ft. (Figure 7).
Figure 7. Milestone applied in the fall showing a small amount of regrowth. Picture taken 200 days after fall applications.
More work needs to be done on this plant to better understand its reaction to a combination of control strategies. Although there were products such as Habitat® and Milestone® that showed promising results, these products required mowing for the full benefit. Fall applications of Habitat® on mowed plots provided the best control at 200 days after treatment; however when applied over the top of unmowed scouringrush it did not reduce biomass at 42 days after application. Milestone® and Habitat® are not labeled for row crops and have substantial rotation restrictions for the planting of some row crops (Figure 7). This may require that the area being treated would have to be taken out of production for the restricted amount of time required by the label.
Reference
1. D. Cloutier and A.K. Watson. 1985. Growth and regeneration of field horsetail (Equisetum arvensis). Weed Science 33:358-365.
2. G. Nice and P. Sikkema. 2007. The Ancient Horsetail. WS-29-W.
3. R.J. Richardson and B.H. Zandstra. 2004. Equisetum Control. Accessed July 19, 2010. <http://www.ipm.msu.edu/landreport/2004/equisetumControl.pdf>.
Sudden Death Syndrome in Soybean – (Kiersten Wise)
Sudden death syndrome, or SDS, has been observed in soybean fields in Indiana over the last week. The fungus that causes SDS, Fusarium virguliforme, infects soybean early, and symptoms are typically expressed later in the growing season. Many soybeans throughout Indiana sat in wet soils this spring before emergence, and growers should be watching for symptoms of SDS in fields over the next few weeks.
Symptoms of SDS are expressed as interveinal yellowing and necrosis (Figures 1 and 2). Veins of symptomatic leaves will remain green. Leaflets will curl or shrivel and drop off with only the petiole remaining attached. If symptomatic plants are pulled from the soil and split down the stem, the lower stem will have a dark or discolored cortex, while the pith will remain white or light brown.
SDS is a disease that is best managed through preventative methods. Producers are encouraged to plant varieties that are less susceptible to SDS in fields with a history of the disease. SDS is typically more problematic in early-planted soybeans. Planting fields with a history of SDS last may reduce the risk for SDS. Foliar fungicide applications are not recommended for management of SDS.
Figures 1 and 2. Foliar symptoms of sudden death syndrome (SDS) on soybean leaves.
Figures 1 and 2. Foliar symptoms of sudden death syndrome (SDS) on soybean leaves.