Pest & Crop Newsletter, Entomology Extension, Purdue University
- Raining Black Cutworm Moths
- Corn Feeding This Early Not Black Cutworm
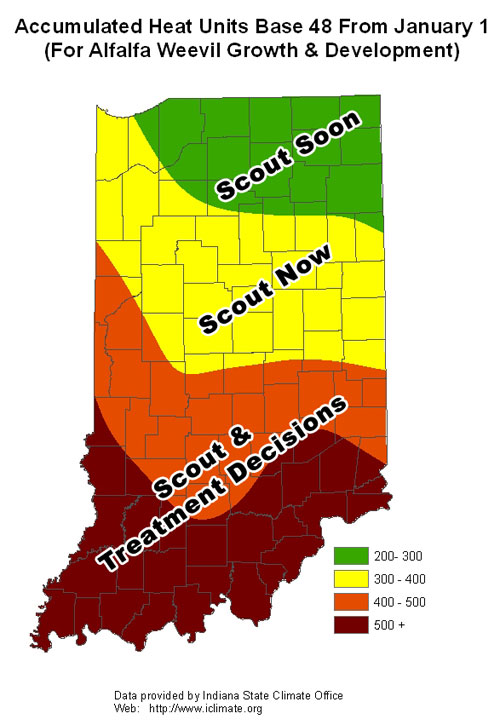
- Accumulated Heat Units Base 48
- Black Cutworm Adult Pheromone Trap Report
- Black Light Trap Catch Report
![]()
Raining Black Cutworm Moths - (Christian Krupke, John Obermeyer, and Larry Bledsoe)
- Recent storms brought many black cutworm moths.
- Timing of scouting can be improved by tracking heat unit accumulations.
- Scouting fields and treating when necessary makes more sense than the preventative applications of insecticides.
- Don’t rely on insecticide-treated seed alone to prevent economic damage.
Most of our dutiful trapping cooperators throughout the state captured black cutworm moths this past week, including four intensive captures, i.e., 9 or more moths caught over 2-nights. This recent flush of moths is attributed to the major weather front that moved through the Midwest last week. In referring to the following weather map graphic, it is quite evident how black cutworm moths in Mexico and the Gulf States were caught up in this powerful storm moving from the Southwest and “delivered” to the Midwest. Immediately following the front passing, pheromone trap captures increased.
Significant moth captures at this time, followed by the use of heat units to predict the beginning of larval activity, gives us an indication of potential severity of the problem and locations of concern. With this information we are able to predict with some degree of accuracy when and where crop damage is likely to occur based on these data. Refer to future issues of the Pest&Crop as we track heat unit accumulations and predicted damage in your area.
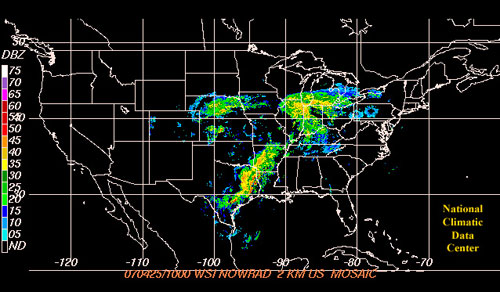
April 25 storm from the Southwest

Black cutworm moths on pheromone trap bottom
![]()
Corn Feeding This Early Not Black Cutworm - (Christian Krupke, John Obermeyer, and Larry Bledsoe)
- There are many species of cutworms that nibble on corn.
- Black cutworm is the most common and damaging species.
- The dingy cutworm is a “climbing cutworm” and rarely cuts plants.
- The dingy cutworm overwinters as a partially grown larva.
- Proper ID between black and dingy cutworms is important, see the aides below.
Many cutworm species look alike and identification is often confusing. Proper identification is critical because the black cutworm can be an economic threat to corn, whereas other species are typically not. As the name suggests, the black cutworm will cut or burrow into plant stems, causing stand losses. The black cutworm is our most commonly found species, but some fields will have a mixed bag of dingy, claybacked, and variegated cutworms.
Black cutworms do not overwinter in the Midwest, which is why we monitor their arrival each spring with pheromone traps. Once they arrive in large numbers (intensive captures) we begin predicting their development and subsequent damage with heat unit accumulations. There have not been enough heat units accumulated this spring for black cutworm to get 1/2 to 3/4 inches long – the size when they begin to cut plants.

Early black cutworm leaf feeding
The dingy cutworm, probably the second most common species, is primarily a leaf feeder and will rarely cut plants, and if it does, the cutting is above ground level. Because a corn plant up to the 5-leaf stage can withstand severe defoliation without a yield loss (compare it to frost damage), treatment for the dingy cutworm is rarely justified. To confuse the issue even more, there are many other species that one may find while scouting. For example, the claybacked cutworm is not as common as the black and dingy, and its damage is a mix of leaf feeding and plant cutting. The dingy and claybacked cutworms overwinter as partially grown larvae, therefore finding cutworms 3/4 of an inch or more at this time would likely point to these species.

Dingy cutworm leaf feeding
Below are some morphological characteristics that may help differentiate the black and dingy cutworm species.
Identification Features:
- do not use color!
- skin textures are different (black = granular, dingy = smooth)
- tubercle (black warts or bumps) size on the top center of the body segments are different: the black’s outside pair are about twice the size of the inside pair, the dingy’s are all about the same size. Refer to the following diagram.
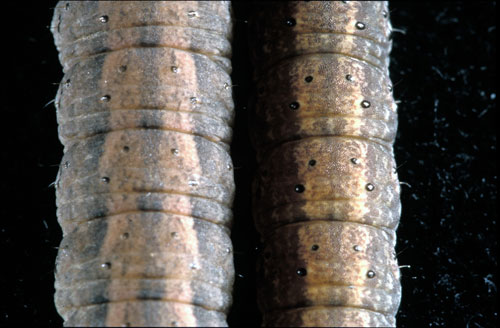
Dorsal surface of dingy (left) and black cutworm (right). Note tubercle sizes.
![]()
Click here to view the
Black Light Trap Catch Report - (John Obermeyer)
Soil Bioassays and Crop Rotation - (Glenn Nice and Bill Johnson)
There are several herbicides which provide soil residual activity. This can be a benefit in controlling weeds with extended germination patterns to reduce competition, and provide flexibility in the timing of postemergence applications. Soil residual herbicides can also often provide more consistent season long weed control compared to total postemergence weed management programs. However, in some cases due to environmental and soil characteristics, and the physical and chemical properties of the herbicide, these herbicides can persist after the growing season of the crop that they were used in and injure rotational crops. When this occurs it is termed persistence or “carryover.”
Soil pH can have a large influence on the persistence of herbicides in soil. Some herbicide labels have specific instructions on use rates based on soil pH. One example of this is a herbicide that contains chlorimuron (Classic, Canopy products). Chlorimuron can be applied at 1/4 oz/A if the pH is not known in medium to fine soils; however, if the composite soil pH is 7 or below, chlorimuron can be applied up to 3/4 oz ai/A in a medium or fine soil. Chlorimuron is more persistent (i.e. breaks down slower) in high pH soils and can injure rotational crops such as corn.
Soil pH can also effect how well a herbicide binds to soil particles, making the herbicide unavailable and reducing weed control. At low pH, atrazine is more tightly adsorbed to soil and thus atrazine residues can remain in soil for longer periods of time. In other cases changes in soil pH can result in less binding to soil, and make the herbicide more available in the soil solution to plants and cause injury to the crop. Table 1 lists a few more examples of how soil pH can effect herbicides.
Table 1. Herbicide and soil pH interactions. For a more compete table see the 2007 Weed Control Guide for Ohio and Indiana. |
|||
Herbicide |
Soil pH |
Influence |
Result |
| Atrazine | < 5.5 |
Adsorbed to soil | Reduction in weed control |
| Atrazine | > 7.0 |
Released into soil | Better weed control; possible carryover |
| Balance Pro | > 7.5 |
Degradation is slowed | Better weed control; possible carryover |
| Hornet WDG | > 7.8 |
Released into the soil | Possible increase of crop injury |
| FirstRate | > 7.8 |
Hydrolysis slowed | More available for carryover |
| Sencor | > 7.0 |
Released into the soil solution | Possible increase of crop injury |
| Taken from the 2007 Weed Control Guide for Ohio and Indiana (http://www.btny.purdue.edu/Pubs/WS/WS-16/HerbSoilpHInter06.pdf) | |||
Crop rotation restrictions are established to insure enough time has passed to allow the decomposition of the herbicide to levels that won’t injure rotational crops. Some crops are more sensitive to small amounts of the specific herbicide and require longer rotational intervals. An example of this is sugar beet and many of the ALS herbicides. There is a 40-month rotation restriction for sugar beet following Lightning, and a 30-month restriction following FirstRate or Gangster. For a list of rotation restrictions in some of the crops planted in Indiana and Ohio see the 2007 Weed Control Guide for Ohio and Indiana, table 22 <http://www.btny.purdue.edu/Pubs/WS/WS-16/HerbCropRot.pdf>.
Soil and environmental conditions are highly variable. This variability makes it difficult to insure that trace amounts of the herbicide are gone in all situations. For highly sensitive rotational crops, many labels recommend bioassays before planting even after the rotational interval. Table 2 lists a few herbicides that have labels that require bioassays to be done before rotating to specific crops.
Table 2. Herbicide labels that indicate that bioassays should be considered before specific rotational crops are planted. |
|||
Herbicide |
Crops |
Herbicide |
Crops |
| FirstRate | oats, clover, sugar beet, tomatoes | Hornet | clover, sugar beet, tomatoes, |
| Equip | clover, tomatoes | Lightning | clover, sugar beet, tomatoes, |
| Gangster | alfalfa, clover, sugar beet, tomatoes | Python | clover, sugar beet, tomatoes, |
A bioassay is the use of a living organism as an indicator species to determine presence or concentration of an organic substance which can influence growth of the indicator species. In my undergrad years I conducted an experiment that used an algae species to quantify concentrations of atrazine in lake water.
A bioassay for our purposes would be to test if levels of herbicide were still present in a field that could injure a susceptible rotational crop. The easiest way to perform a bioassay in this case would be to collect soil from the area that has been treated with the herbicide in question (suspect) and soil from an area that has not been treated with the herbicide (control). It is important that the control is soil from a non-suspected area, but is a similar soil type. The control is to help confirm if the injury you are seeing is truly due to something in the soil and not the fact that you may not have a green thumb. The samples from the suspected area and control area should be kept separate. Take soil samples from the top 3 inches of soil. Soil samples from various areas of the suspect area can be mixed. You will want to plant seeds of your rotational crop in both the suspect and control soil. Let the plants germinate and be sure to provide them with plenty of sunlight and water. If injury symptoms appear on plants in the suspect pots and not in the control pots, it would indicate that the herbicide was still present at levels that will cause damage. If the same injury symptoms occur on the control, this would indicate that there is some other influence causing the problem and would make the bioassay inconclusive.
A bioassay can take a couple of weeks before a result is given, so it would be a good idea to initiate these at least one month before planting. Too soon and you may be in the rotation restriction, not giving the herbicide a fair chance to break down, too late and you may not get a result quick enough to make a alternative plan.
Although it may seem rotational restrictions are in place to give us practice at counting off months and bioassays are troublesome, they can be used to avoid crop loss due to herbicide carryover. Remember a little aggravation up front by performing a bioassay is far better than watching the development of a ten or hundred acre bioassay develop.
1 M.M. Loux, J.M. Stachler, W.G. Johnson, G.R.W. Nice, and T.T. Bauman. 2007. Herbicides and Soil pH Interactions. Weed Control Guide for Ohio and Indiana. p.72. http://www.btny.purdue.edu/Pubs/WS/WS-16/HerbSoilpHInter06.pdf
Requirements for Uniform Germination and Emergence of Corn – (R.L. (Bob) Nielsen)
Rapid, uniform germination and emergence of corn help set the stage for maximum grain yield at the end of the season. Without such a successful start to the season, the crop is behind the proverbial “eight-ball” right from the beginning. The good news is that there are only four simple requirements for uniform germination and emergence of corn. The bad news is that one or more of the requirements are sometimes absent in one field or another.
Adequate and uniform soil moisture at the seed zone.
Adequate soil moisture is most simply defined as not too dry and not too wet. Most growers know what “adequate” looks and feels like. Uneven soil moisture in the seed zone can be caused by variable soil characteristics, tillage patterns, unusual weather conditions and uneven seeding depth. Uneven soil moisture throughout the seed zone is the primary cause of uneven emergence, the results of which can easily be yield losses of 8 to 10 percent. Remember that uneven seedbed soil moisture can be described as “adequate’ versus “too wet” as well as “adequate” versus “too dry.”
Useful Tip: When seedbed conditions are dry, make sure that your choice of seeding depth ensures uniformly adequate soil moisture for the germination of the seed. Even though a 1.5 to 2 inch seeding depth is a good choice for many conditions, don’t hesitate to increase seeding depth to 2.5 to 3 inches if that is the depth where the uniform soil moisture is located. Planting shallower than 1.5 inches increases the risk of poor or uneven germination during subsequent drying of surface soils.

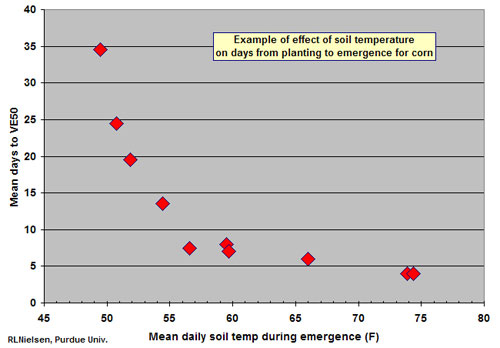
Adequate and uniform soil temperature at the seed zone.
Corn will germinate and emerge slowly and unevenly when soil temperatures are less than 50F. When soils warm to the mid-50’s or warmer, emergence will occur in seven days or less if soil moisture is adequate. Thermal time from planting to emergence is approximately 120 growing degree days (GDDs) using the modified growing degree formula (Nielsen, 2007b) and air temperatures or about 130 GDDs if using soil temperatures.
Uneven soil temperature in the seed zone can be caused by variable soil texture, soil color, soil drainage, surface residue cover in reduced tillage systems and seeding depth control. Temperature variability during germination and emergence is most critical when average soil temperatures are hovering about the critical 50F minimum threshold value.
Useful Tips: Dark-colored soils will typically warm more quickly than light-colored soils. If soils dry differently across the field, the drier areas will typically warm faster than the wet areas. Uneven residue cover (surface trash) in reduced tillage systems causes significantly lower soil temperatures under the heavier cover than under barer spots in the field. Uneven seeding depth exposes deeper planted seeds to slightly cooler seed zones than seeds placed shallower. Consider row-cleaning attachments for the planter to move aside the surface trash during planting and expose the seedbed to sunlight and its warming effects. Consider strip tillage practices in the future to better manage surface trash in a reduced tillage system.
Adequate and uniform seed-to-soil contact.
In order for the kernel to absorb moisture quickly and uniformly, soil must be firmed completely around the kernel. Seed-to-trash contact results from “hair-pinning” of surface trash into the seed furrow during no-till planting when soil and/or trash are too wet for adequate coulter cutting action. Seed-to-clod contact results from planting into cloddy fields created by working soil too wet. Seed-to-rock contact is, needless to say, not good for proper germination either. Seed-to-air contact results from open planter furrows when no-till planting into excessively wet soils. Germination of kernels lying in open planter furrows is dependent on rainfall keeping the open furrow environment moist.
Useful Tips: Whippers, wipers, movers, fingers, and other similar trash management gadgets for the planter are most beneficial when you are challenged with rocky, cloddy, or trashy surface soil conditions. They help clear the way (literally) for the planter’s double-disc openers to more easily do their job of creating an optimum seed furrow. Other planter attachments that help press the kernels into the seed furrow can improve seed-to-soil contact and seeding depth uniformity when seedbed conditions are otherwise challenging.
Surface Soil Free From Crust.
Severe surface crusting or compaction will restrict emergence of the coleoptile and cause underground leafing or plant death. Severe sidewall compaction can also limit elongation of the mesocotyl and emergence of the coleoptile.
Useful Tip: Avoid excessive tillage prior to planting the crop, especially if significant rainfall is forecast prior to emergence of the crop. Avoid excessive downpressure on the closing wheels of the planter. Avoid planting “on the wet side” that often results in smeared sidewalls.
Related References
Carter, Paul, Emerson Nafziger, and Joe Lauer. North Central Regional Extension Pub. No. 344. [On-Line]. Available at <http://learningstore.uwex.edu/pdf%5CNCR344.pdf.> (URL verified 4/23/07).
Jasa, Paul. 2007. Avoiding Sidewall Compaction at Planting — Don’t Plant Too Shallow. CropWatch Newsletter, Univ. of Nebraska. [On-Line]. Available at <http://cropwatch.unl.edu/archives/2007/crop8/sidewall_compaction.htm>. (URL verified 4/23/07).
Nielsen, RL (Bob). 2007a. Germination Events in Corn. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/timeless/GerminationEvents.html>. (URL verified 4/23/07).
Nielsen, RL (Bob). 2007b. Heat Unit Concepts Related to Corn Development. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/timeless/HeatUnits.html>. (URL verified 4/23/07)
Nielsen, RL (Bob). 2007c. The Emergence Process in Corn. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/timeless/Emergence.html>. (URL verified 4/23/07).