Pest & Crop Newsletter, Entomology Extension, Purdue University
- Winter Temperatures, Corn Flea Beetle Survival, and Potential for Stewart's Wilt
- SWCB Spring Survey Update
Winter Temperatures, Corn Flea Beetle Survival, and Potential for Stewart’s Wilt - (John Obermeyer, Christian Krupke, and Larry Bledsoe)
- Corn flea beetle winter survival is expected to be low in northern Indiana.
- Moderate survival is expected for most of southern Indiana.
- Extreme southwestern counties of the state may have high survival.
- Snow cover in February may have benefited some overwintering beetles.
- Corn flea beetle is a vector of Stewart’s wilt of corn
- Management guidelines are given below.
Corn flea beetle is a sporadic pest in Indiana. Winter temperatures in regions where beetles were abundant last season will determine if there is cause to be concerned this season. This is especially important since this insect transmits the bacterium that causes Stewart’s disease in corn. The severity of the disease correlates well with winter temperatures because the organism survives in the gut of the overwintering beetles. Warmer temperatures result in higher beetle survival, and therefore a greater potential for Stewart’s disease. To determine the potential severity of Stewart’s disease, add the average daily temperatures for the months of December, January, and February. If the sum is below 90, the potential for disease problems to develop is low. If between 90 and 100, moderate disease activity is a possibility. Sums above 100 indicate a high probability that severe problems will develop for susceptible corn. To help you better gauge the potential for corn flea beetle activity in your area (and the potential severity of the threat of the disease), we have created the state map shown below. According to the temperature model, there is low probability of corn flea beetle activity and subsequent disease in northern Indiana, and moderate activity in areas south of Interstate-70 to just north of the counties in extreme southwestern Indiana. Conditions were very favorable in the extreme southwestern counties for beetle survival, and this could result in the appearance of Stewarts’s wilt in sensitive hybrids/inbreds/sweet corn this spring.
This temperature model for corn flea beetle has been in use for many years and has been fairly accurate in predicting the activity of this pest the following spring. However one inherent flaw is that the model is based on ambient air temperatures, not temperatures under leaf litter and grass clumps where this pest is actually overwintering. If snow cover is present, this provides an insulating blanket for the insect, and may protect some beetles from winterkill. Even with this “disclaimer” statement, we think the 2006/2007 winter was cold enough to have negatively impacted overwintering beetles in northern Indiana. Also, flea beetle numbers have been low statewide, in general, for the last couple years.
As for the disease, there are two phases of Stewart’s disease: a wilt phase and a leaf blight phase. In the wilt phase, plants wilt rapidly, usually at an early stage of growth. Leaves emerging from the whorl of infected plants are often the first to wilt. Internal tissues at the growing point are discolored or hollowed out. Faint green to yellow streaks containing corn flea beetle feeding marks are visible on one or more leaves. If stalks of wilted plants are cut, it may be possible to see yellow, moist beads of bacterial ooze. Sweet corn hybrids are especially susceptible. Some dent corn inbreds, and occasional dent corn hybrids, and some popcorn lines are susceptible as well. Dent corn hybrids rarely wilt after growth stage V5.
The leaf blight phase can occur at any time during the growing season, but often does not appear until after tasseling. Lesions are long and narrow, with pale green to yellow streaks and irregular or wavy-margins. Streaked areas die and become straw-colored. Severely infected leaves may die prematurely. Lesions on leaves of older plants may be confused with northern corn leaf blight. One way to differentiate the two is that it is usually possible to see beetle feeding tracks in Stewart’s disease lesions.
Management decisions made now should be based on the corn’s susceptibility to the disease and anticipated risk.
Low susceptibility/risk - pest managers should scout fields and apply a foliar rescue treatment after emergence if (1) beetle feeding damage becomes severe, (2) there are 5 or more beetles per plant, and (3) seedlings are growing slowly (e.g., cool temperatures).
High susceptibility/risk - sample field edges and in-field areas of grass weed residue (i.e., overwintering sites) before planting to assess overwintering beetle survival and potential beetle movement to emerging corn plants. A sweep net is an ideal sampling tool for this pest. If any beetles are discovered at this time, an at-planting insecticide application is warranted. Most of the corn seed currently sold in Indiana is already protected from corn flea beetle at the time of purchase: Cruiser and Poncho insecticide-treated seed are systemic insecticides that should give good control of flea beetle in the early seedling stage. The low rates of the seed treatments are expected to provide protection from emergence to 2-leaf corn, whereas the higher (rootworm) rate should protect corn through the 5th leaf stage.
If insecticide-treated seed is not an option, foliar insecticides broadcasted at the time when corn spikes should provide 7 to 10 days of residual protection from beetle feeding.
CAUTION: treating of field edges and waterways for beetle control may be an off label application. Avoid movement of insecticides, including soil-bound materials into aquatic environments.
| Expected Flea Beetle Winter Survival |
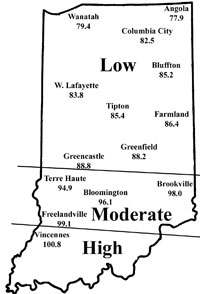 |
| Disease Site | Dec. |
Jan. |
Feb. |
Sum |
Threat |
| Angola | 34.8 |
27.6 |
15.5 |
77.9 |
Low |
| Wanatah | 36.6 |
27.5 |
15.6 |
79.4 |
Low |
| Columbia City | 38.0 |
28.9 |
15.6 |
82.5 |
Low |
| Bluffton | 39.5 |
29.6 |
16.1 |
85.2 |
Low |
| W. Lafayette | 39.1 |
28.5 |
16.2 |
83.8 |
Low |
| Tipton | 38.8 |
29.8 |
16.8 |
85.4 |
Low |
| Farmland | 39.3 |
30.6 |
16.5 |
86.4 |
Low |
| Greenfield | 39.2 |
30.9 |
18.1 |
88.2 |
Low |
| Greencastle | 29.0 |
31.1 |
18.7 |
88.8 |
Low |
| Terre Haute | 40.9 |
32.0 |
22.0 |
94.9 |
Moderate |
| Brookville | 42.8 |
34.9 |
20.3 |
98.0 |
Moderate |
| Bloomington | 4.13 |
33.1 |
21.7 |
96.1 |
Moderate |
| Freelandville | 41.1 |
33.5 |
24.5 |
99.1 |
Moderate |
| Vincennes | 40.2 |
35.0 |
25.6 |
100.8 |
High |
![]()
SWCB Spring Survey Update - (Ric Bessin and Mike Smith, Kentucky Pest News, No. 1119, March 12, 2007)
Southwestern corn borer spends the winter as larvae in galleries at the base of corn stalks. Stubble in cornfields can be checked during early spring for damaged plants and surviving borers. This provide an indication of the level of moth flight this spring and an indicator of what the first generation may be like for 2007. A survey of southwestern corn borer damage and larval survival was conducted in Daviess and Henderson counties on March 12. These counties were selected because of past infestation and sampling history. The purpose was to estimate the extent of SWCB damage in 2006, as evidenced by basal stalk girdling. In addition, we wanted to estimate the survival of the over-wintering larvae in the crowns of these damaged plants. In each county, four non-Bt corn fields were evaluated. Within each field, 10 random groups of 10 consecutive plants were examined for girdling and an additional of 50 (minimum) girdled plants were examined for the presence of live SWCB larvae.
2005 SWCB Spring Survey Results |
||
Location |
Damaged Plants |
SWCB Recovered |
Henderson Co. |
||
4 Farms |
58 / 400 |
5 / 400 |
Daviess Co. |
||
2 Farms |
22 / 200 |
3 / 200 |
The information from Daviess and Henderson counties indicated that there was a moderate incidence of stalk girdling when compared with previous years, but the survival of those larvae was the lowest observed in any of the nine years that we’ve been conducting this survey. As in past years, there were high levels of what appeared as bird predation on the larvae.
Year |
Girdled Stalks (%) |
Survival/Girdled Stalk (%) |
Overall Survival/Stalk (%) |
2007 |
12.8 |
1.4 |
0.18 |
2006 |
15.0 |
31.8 |
4.82 |
2005 |
5.6 |
5.1 |
0.29 |
2004 |
15.6 |
2.5 |
0.39 |
2003 |
26.6 |
4.3 |
1.13 |
2002 |
11.8 |
5.3 |
0.63 |
2001 |
40.6 |
9.7 |
3.92 |
2000 |
20.7 |
26.9 |
5.57 |
1999 |
35.9 |
10.1 |
3.64 |
So for the spring of 2007, we can conclude:
• The ‘normal’ winter has reduced survival of SWCB larvae in the counties surveyed, the numbers are very similar to what was observed in the spring of 2004.
• Birds continue to feed heavily on SWCB larvae during the winter.
• Winter conditions were not sufficient to eliminate SWCB larvae.
• We expect low to moderate first generation SWCB pressure for those areas surveyed.
• Date of planting is still important. Corn planted after May 1 could be at risk to late season SWCB activity.
New Canopy EX Label Allows for Application Closer to Planting – (Glenn Nice, Bill Johnson, and Tom Bauman)
As mentioned in the 2007 Indiana Weed Science Update1, DuPont has been working on a Canopy EX label that would allow applications closer to the time of planting soybean. Before recently, Canopy EX has been labeled for fall and spring applications at least 45 days before planting.
Changes to the Canopy EX label now allow the application of Canopy EX in no-till or conservation tillage fields in the fall and up to 7 to 14 days before planting soybean. Canopy EX at a rate of 1.1 to 2.2 oz/A can be applied a minimum of 7 days before planting. Rates greater than 2.2 and up to 3.3 oz/A require a minimum of a 14 day wait before planting.
In Indiana Canopy EX can be applied at 1.5 to 3.3 oz/A on soils with a composite soil pH of 7 or less. If your soil pH is highly variable or you do not know your composite soil pH, it is required that you use the 1.1 oz/A rate.
Canopy EX is controls several winter annual weeds such as common chickweed, mustards, horseweed (marestail), henbit, and purple deadnettle. As well as providing excellent to good control of several summer annuals such as ragweeds, jimsonweed, pigweeds, common lambsquarter, smartweed, and velvetleaf. The addition of COC at 1% v/v is required and the addition of 1 pt/A 2,4-D is suggested to increase activity and weed spectrum.
1. Nice and Johnson. 2007 Indiana Weed Science Update. <http://www.btny.purdue.edu/weedscience/2007/2007update.pdf>.
![]()
Roundup Ready Alfalfa in the Courts – (Glenn Nice and Bill Johnson)
In a recent court case, the United States District Court in California made a decision that defendants were in “violation of the Nation’s Environmental Protection Act by failing to prepare an environmental impact statement before deregulating Roundup Ready alfalfa1.” This means that the USDA did not prepare an environmental impact statement before deregulating the Roundup Ready alfalfa. The case was presented due to concerns regarding the “potential significant environmental impact of gene transmission.”
Gene transmission, also termed “gene flow,” is a situation where pollen from genetically modified crops move into conventional (non-modified) crops or into closely related wild species. This would be an unintended transfer of the introduced genes into these crops or weeds. Companies that wish to register products or crops that have modified gene sequences have to provide sufficient evidence to the Environmental Protection Agency (EPA) that gene flow is not a concern.
In this case, the court decided that if Roundup Ready alfalfa was already planted that the alfalfa stand could remain in the field. That there would be no prohibition of “harvesting, using, or selling any Roundup Ready alfalfa that has already been planted1.” Furthermore the decision will allow producers who have already contracted or bought RR alfalfa be planted up to March 30, 2007. However, after March 30 Roundup Ready alfalfa can not be planted until the issue is cleared in the courts.
1. Preliminary Injunction Order. March 12, 2007. The United State District Court for the Northern District of California.
Among the things that could cause bees not to return to the hive are Nosema disease which causes dysentery, or tracheal mites, or viruses. In fact, when your bees get typical parasitic mite syndrome and show diseased brood, the colony often dwindles without many dead bees in the hive, although you often see bees crawling around with deformed wings (probably caused by deformed-wing virus). Where did all the other bees go? Last year, it seems there was rapid dwindling of bee hives in fairly large areas. The press release originating from Penn State has gotten a lot of play. The preliminary analyses of samples showed a significant amount of Varroa in hives that had dwindled, suggesting that maybe mites were a factor. They also saw various confusing symptoms in the dead bees. There is definitely something going on, but it may not be something new. Nosema, or dysentery disease is caused by Nosema apis (a spore-forming protozoan). In Europe they recently found Nosema cerana associated with dying hives. Like Varroa mites, N. ceranae came from the Asian honey bee, Apis cerana. It is controversial whether N. cerana is the cause of those colony deaths in Europe. I recently talked to a virologist (Judy Chen) at the USDA bee lab in Beltsville and she had checked for N. cerana in the U.S. and said that it was common. Tom Webster at KSU also found N. ceranae in samples said to have CCD. We don’t know how long this pathogen has been in the U.S. because nobody tested for it before! Maybe N. ceranae is a factor in CCD. Nobody knows. Nosema is considered a minor pathogen of bees but, fall application of Fumagillin (Fumadil-B) in sugar syrup will protect your bees from both kinds of Nosema that could weaken your hives.
In 2006, CCD was primarily a problem of migratory beekeepers. Moving bees causes them to be stressed, especially when they do not have good food sources. Devon Howald from Huntington, along with Dave Shenefield of LaFountaine took about 1500 hives from IN to CA and some of them dwindled while waiting for the almonds to bloom. But the ones that had access to good nectar were OK. They hope to at least break even after spending a lot of money in freight costs. Four out of five migratory beekeepers they spoke to in CA were seeing dwindling hives. The press release that generated this “buzz” included data from a survey by Bee Alert Technologies which showed CCD was a problem in 25 states on a map. However, some of the states on that map did not report widespread symptoms of CCD. They originally said IN was affected, but have updated the map. See <http://cyberbee.net/ccd.html>, for Zachary Huang’s CCD page.
The problem we had in Indiana this winter was no fall nectar flow and those who did not have time to feed their bees lots of sugar syrup early enough in the fall had colonies that starved. Clover Blossum Honey Company in LaFountaine may have 50% losses in many areas due to starvation. They can split their hives and make this up, but that is a lot of work. I am guilty of allowing starvation to take half of our Purdue hives. Leaving your supers on until September helps when there is a poor fall flow because it allows your bees to draw some of the honey from the supers down into the brood nest, but if there is no nectar flow in the fall they may still need syrup. My opinion is that there is no reason for beekeepers to worry about mysterious ailments. Hopefully, researchers will be able to provide answers. We beekeepers should monitor our hives for Varroa mites and control them when they get too high, preferably with “soft” chemicals, and we need to try to find bees that can tolerate the mites (see <http://www.entm.purdue.edu/beehive/>, E-201-W “Parasitic Mites of Honey Bees”). In the fall and early spring, we should check our bees and feed if necessary. If we do these things, our bees will be OK. If you move your bees around for pollination, you will have to be careful with their nutrition and you may have to take losses sometimes. Hobby beekeepers are responsible for a large proportion of the pollination services of honey bees and an important part of agriculture.


