Pest & Crop Newsletter, Entomology Extension, Purdue University
![]()
Soybean Aphid Update – (Christian Krupke, John Obermeyer, and Larry Bledsoe)
Aphid numbers remain low across most of the Midwest, and we still have no fields reported over threshold in Indiana. All current information indicates we may get off lucky this year, but until the beans have reached the pod-fill stage, we won’t sound the “All clear.”
The highest concentrations of aphids are still in the northeastern counties and producers in these areas should drop by and take a quick look at fields, if they have not already. The lower humidity and cooler-than-average temperatures will help aphid populations build, so stay vigilant.

Have you checked your soybean field for aphids lately?
![]()
Rain and Spider Mites – (Christian Krupke, John Obermeyer, and Larry Bledsoe)
- Rainfall has little direct impact upon spider mites.
- Rainfall does encourage plant growth and vigor - this is the key.
- Depending on future weather, rain may be a brief respite to spider mite damage and spread.
Scattered thunderstorms last week have brought moisture relief to some parched areas of Indiana. Now the question concerning spider mites is whether the rain “controlled” them. Before attempting to answer the question, let’s review the factors that come together to create a spider mite problem in soybean fields.
Extended hot and dry conditions will:
- encourage the movement of spider mites from drying field sides to soybean
- favor rapid (explosive!) reproduction of spider mites
- cause spider mites to increase their feeding
- dramatically reduce fungal pathogens that help keep spider mites in check
- create moisture-stressed plants that provide a higher concentration of nutritious fluids (“protein broth”)
A significant rainfall (1 inch or more) followed by high humidity will:
- recharge the plant’s fluids, making it less conducive for spider mite population growth
- encourage the growth, development, and dissemination of beneficial fungal pathogens
- physically dislodge and/or kill some mites from the leaf surface
Remember, rain does not make the spider mites go away! They are always present in and around every soybean field in the state. They just remain a non-pest until weather is hot and dry for an extended period. If mites are an issue in a field, don’t delay treatments - remember that spider mite damage is irreversible. A brown leaf will not turn green once mites are gone, and brown leaves contribute nothing to yield. Refer to last week’s Pest&Crop for control materials and application techniques.

Microscopic view of a healthy and diseased spider mite.
![]()
Bug Scout
And you thought western bean cutworm could have caused the damage!
![]()
Click here to view the
Black Light Trap Catch Report - (John Obermeyer)
![]()
Japanese Knotweed (Polygonum cuspidatum) – (Glenn Nice)
Several times a year I get calls regarding Japanese knotweed. If you don’t have this plant, consider yourself lucky, but if you do and want to get rid of it, it is going to be a battle.
The Short Story
Japanese knotweed is a perennial that can form large colonies. Its stems can reach up to 10 feet tall and resembles bamboo, although it is not. Control is difficult and requires patience. Small plants can be dug up, make sure that all of the root and rhizome parts are excised, bagged, and disposed of. The active ingredient glyphosate and triclopyr have been reported to have an effect on Japanese knotweed as a stump cut or foliar applications. Foliar applications of glyphosate should be applied when fully leafed out and before bloom. For more detail, read on.
The Longer Story
Japanese knotweed is also referred to as Japanese bamboo, although it is not a bamboo at all, but a member of the buckwheat family (Polygonaceae). It gets its association with bamboo because of the stem’s bamboo appearance. Japanese knotweed is believed to have been introduced into the US from Asia as an ornamental and is now on many states’ noxious and invasive plant lists. It tends to grow wild where soils have been disturbed, often found in ditches, along roadsides, railway tracks, and near drainage topography.
Identification
This plant is a rhizomatous perennial that spreads primarily through vegetative means and forms large thick colonies1. Its thick hollow stems can reach a height of 6 to 10 feet tall, and can be a reddish brown color. As a member of the Polygonaceae family, Japanese knotweed has the family specific character of an ocrea. An ocrea is a membranous sheath that surrounds the stem at the nodes. In Japanese knotweed, the ocrea is often short to missing. Only a thin red ring around the stem can been seen on older plants (Figure 1). Its leaves are four to six inches long, having smooth margins, and come to a point (Figure 2 and 3). Greenish-white or cream flowers appear in the late summer and fall on open panicles2. Japanese knotweed is dioecious, meaning that there are separate male and female plants.

Fig. 1. Japanese knotweed stems showing the red ring of a ocrea

Fig. 2. Japanese knotweed leaf

Fig. 3. Japanese knotweed leaves alternately arranged
Spread
A Japanese knotweed rhizome can extend up to 30 feet from the parent plant, and small fragments can give rise to new colonies by being moved mechanically or by moving water. Dr. J. Bailey of the University of Leicester called the Japanese knotweed “the largest female,” reporting that genetic research suggests that all the plants in the UK were clones of a single introduced female plant3. Of the plants that I have inspected, I have yet to see seed produced. Due to its distribution in Indiana, male and female plants may not commonly come in close enough contact with each other to produce seed. Although vegetative reproduction appears to be the most common means of spread, it can also spread by seed. Research done by Bram and McNair looking at germination of Japanese knotweed seed reported that germination increased from 10 to 90% from seed collected from September to November4.
Uses
Some reported cases from landowners in Indiana mention that it was introduced from suspected landfill or purchased soils, in most cases it is suspected that it was planted as an ornamental. As mentioned above, Japanese knotweed was introduced into the US most likely for use as an ornamental. Its persistence, methods of distribution, and aggressive nature makes the plant difficult to contain. However, Japanese knotweed has also been identified as a source of trans-resveratrol, a compound obtained from grapes, wine, soy, and peanuts. Trans-resveratrol has been connected to slowing bone loss and having antioxidative, anticarcinagenic, and antitumor properties5. In Asia, the root is dried and infused into a tea called Itadori tea. The word Itadori means “well-being” in Japanese5.
Control:
Many people have cut back Japanese knotweed only to watch it come back with a vengeance. Continuous cutting or mowing can deplete the underground rhizomes over several years. The specimen that we have at the weed garden is kept contained by regular mowing around the plant. However, it should be mentioned that our specimen is planted in a three foot deep plastic drainage pipe and this may inhibit the movement of rhizomes. The digging up of small plants can be accomplished, but if any portion of the root system is left behind a new colony can grow back. If dug up, stems and roots should be bagged on site to ensure that they don’t end up in your neighbor’s yard. The use of plastic or poly-tarps or liners can suppress this plant, but if you use this method of vegetation control, buy the thickest you can find for there are some cases where Japanese knotweed has punched through. According to David Beaulieu of “About.com,” the Japanese refer to this plant as “strong plant.6”
There are a limited number of herbicides that have an effect on Japanese knotweed. Those that do will have to be applied several times and sometimes over more than one year for complete control. Even after control is thought to be achieved, regular inspection is required to assure that it is not coming back. Always read and follow herbicides labels when using herbicides.
Glyphosate is the active ingredient in many herbicides including Roundup®, Touchdown®, Rodeo®, and Glypro® to name a few. Glyhosate can be used in a stump cut application, where the stems are cut and the herbicide is applied within a half hour of cutting, or a foliar application. In a stump cut application, cut stems a couple of inches from the ground, then within half an hour apply a 25% v/v solution over the cut stems. New growth can be expected so it should be followed by a foliar application. Foliar applications can be applied at a 1.5 to 2% v/v solution directly to the leaves7. A surfactant may be required depending on the specific glyphosate product used; see specific label for details. Foliar applications should be applied when the plant is fully leafed out, but before bloom. If applying close to water use a herbicide labeled for this purpose; Rodeo® is one example.
The active ingredient tryclopyr has also been reported to be effective on Japanese knotweed. Tryclopyr can be found in the products Crossbow® or Garlon®. Like glyphosate, it can be applied at a cut stump application at 25% v/v, or 2% v/v foliar7. However, Garlon® should be mixed with a basal oil. Tryclopyr and glyphosate will injury or control desirable plants, apply with some precision to avoid contact with desirable plants.
There are several surveys underway to identify possible biological control agents8. However, none of the investigated natural antagonists have made it to the release stage. There are several insect herbivores reported to feed on Japanese knotweed. I personally have seen Japanese beetles go to town on the plant we have at the agronomy farm, but not enough to control it and Japanese beetles are a pest in their own right. Besides, Japanese beetles will eat anything.
References
1) Japanese Knotweed Alliance. 2007.
<http://www.cabi-bioscience.org/html/japanese_knotweed_alliance.htm>
2) Britton, N. and A. Brown. 1913. An Illustrated Flora of the Northern United States and Canada. 2nd ed. Vol 1. p. 676.
3) Bailey, J. Accessed 7/20/07. Research on Japanese knotweed by Dr. John Bailey of the University of Leicester.
<http://www.t-c-m-rd.co.uk/cms_misc/articles/Japanese_Knotweed_by_Dr_J._Bailey.pdf>
4) Bram, M.R. and J.N. McNair. 2004. Seed germinability and its seasonal onset of Japanese knotweed (Polygonum cuspidatum). Weed Science 52:759-767.
5) Burns, J., T. Yokota, H. Ashihara, M.E.J. Lean, and A. Crozier. 2002. Plant foods and herbal sources of resveratrol. Journal of Agricultural and Food Chemistry. 50:3337-3340.
6) Beaulieu, D. Accessed 7/20/07. Killing Japanese knotweed (Polygonum cuspidatum). “About.com” <http://landscaping.about.com/cs/weedsdiseases/a/knotweed_2.htm>
6) Plant Conservation Alliance, Alien Plant Work Group. 2007. Least wanted: Japanese knotweed. <http://www.nps.gov/plants/alien/fact/pocu1.htm>
7) Shaw, R.H., and L.A. Seiger. Accessed 7/20/07. Japanese knotweed. <http://www.invasive.org/eastern/biocontrol/12Knotweed.html>
![]()
The “Don’t Touch Me Plants” – (Glenn Nice)
There are several plants that can cause irritation when touched. These are also considered toxic plants and should not be ingested. The most common of these is the well-known poison ivy, but it is a bit of a challenge for most people to tell the differences between poison ivy and poison oak. If you spend time in the woods or gardening in the yard, it would be beneficial to know which plants are the “don’t touch me plants.” This article looks at several of the plants in Indiana that can produce problems when touched. Some of you may already have experienced the itch of poison ivy or the burning and welting of stinging nettle.
Poison Ivy and Poison Oak
The telltale sign that you could be looking at poison Ivy (Toxicodendron radicans - eastern poison ivy or T. rydbergii - western poison ivy, Indiana has both) or Atlantic poison oak (T. pubesens) is the characteristic trifoliate leaf. The two opposite leaflets will have very little or no petiole and the terminal leaflet will be extended from the two opposite leaflets (Figure 1 and 2). There are other plants that might have a similar leaf1, but it might be wise to treat everything with this leaf arrangement as potential poison ivy. According to the USDA plant database, poison oak has not been reported in Indiana as of yet, but it has been reported in Illinois2. However, although the USDA has not officially confirmed poison oak in Indiana, I have received word-of-mouth reports that it is present.

Figure 1. Poison ivy trifoliate leaf. Two leaflets close to the rachis with one at the tip of the rachis. (Photo Source: M. Ross, Purdue University)

Figure 2. Poison oak trifoliate. (Photo Source: Robert H. Mohlenbrock. 1991. Southern wetland flora: Field office guide to plant species. South National Technical Center, Fort Worth, TX)
Poison ivy can grow as a vine or low shrub. It will climb trees, power line poles, fences or just about anything available to climb. Poison oak is a shrub with hairy leaves that have an oak-leaf appearance, hence the name. Both plants produce greenish-white berries which birds eat, spreading the seed. In the winter time, the berries still have the oils that cause dermatitis so they need to be avoided also. Decreases in cases of poison ivy in the winter time is most likely due to the extra clothing worn to fight the cold. The compound that causes all the trouble is urushiol oil or toxicodendrol3. Although sensitivity to urushiol oil can be different for different individuals, very small amounts are generally required to cause a rash. Sensitivity to the oils can also change as a person gets older. Although I have spent a fair amount of time in the woods and have come in contact with poison ivy, I have yet to experience the displeasure of the rash, yet this may change one day. Other people that I know are often fond of saying, “If I even see the stuff I break out in a rash.” The oils can last for a long time on surfaces. The use of water alone will not remove the oils and in some cases water alone can spread the oils. To remove the oils wash skin or clothes with an alkaloid soap. Alcohol will also remove oils2. There have been many proposed treatments of poison ivy dermatitis. In the “Peterson Field Guide to Edible Wild Plants” it mentions that washing with Jewelweed (Impatiens capensis) juice can be used as a home remedy4. A 1902 report in The American Journal of Nursing, it mentions, “…bathing the affected parts with a solution of sugar of lead (lead acetate), with the addition of laudanum5.” I can see where the laudanum (an opiate) would reduce the discomfort, but I would not attempt this at home. The same report mentions turning to a solution of water, alcohol, witch-hazel, and ammonia5.
Poison Sumac
A shrub or small tree, poison sumac (T. vernix) can be more toxic than poison ivy4 (Figure 3). Plants can grow from six to 20 feet tall. The compound leaves have seven to 13 leaflets with smooth margins and the leaflets are elliptic to oblong. Flowers are green and approximately 1/16 of an inch in diameter. There are other sumacs in the state of Indiana that are not toxic. Although poison sumac has been moved to the Toxicodendron genus, the other sumacs remain in the Rhus genus. The USDA plant database reports fragrant, winged, smooth, Northern smooth, and staghorn sumac in Indiana and the surrounding states2. These are often used for landscaping. The number of leaflets are often the same between the toxic poison sumac and its nontoxic counter parts. One way to help identify between the nontoxic sumacs and poison sumac is that the berries of poison sumac are hairless, drooping, and are green when immature, but then turn grey-white as they mature. The berries of other non-toxic sumacs often have hairs on the berries and are red to crimson6. Another way poison sumac can be differentiated from the non-toxic sumacs by carefully looking at the leaves. Poison sumac leaflets are entire and do not have a winged rachis. Non-toxic sumacs either have serrations on the margins of the leaves, like staghorn sumac (Rhus hirta) and smooth sumac (R. glabra) or have entire leaves but with a winged rachis, as in winged sumac (R. copallina).

Figure 3. Poison Hemlock. (Photo Source: Ted Bodner in James H. Miller and Karl V. Miller. 2005. Forest plants of the southeast and their wildlife uses. University of Georgia Press, Athens)
Poison Hemlock
At first glance poison hemlock (Conium maculatum) may appear like wild carrot (Daucus carrota) or some kind of giant parsley, but it is not a mistake that you should make (Figure 4). Although poison hemlock is more known for poisonings as a result of ingesting, for example the death of the Greek philosopher Socrates7,8, the plant’s natural oils may absorb through the skin. So if you find yourself hand pulling poison hemlock, it would be a good idea to wear gloves. Both poison hemlock and wild carrot belong to the parsley family (Apiaceae). Both have the characteristic umbel inflorescence of small white flowers and leaves that expand at the bases sheathing the stems. You can tell poison hemlock apart from its benign cousin, wild carrot, by the presence of purple blotches on the stem. The leaves of poison hemlock are also sharper in detail compared to wild carrot.

Figure 4. Poison Hemlock in corn stuble. (Photo Source: Glenn Nice, Purdue University)
Cow Parsnip and Giant Hogweed
Also members of the parsley family, both these plants can cause a reaction to sunlight called phytophotodermatitis. However, the reaction from giant hogweed (Heracleum mantegazzianum) is more severe than that of cow parsnip (H. maximum), resulting in large blisters and red to purple rashes that can scar. Giant hogweed is NOT common in Indiana; only two plants have been reported to date as of writing this article. However, it has been reported in 11 counties in Michigan2. Cow parsnip, although not that common, can be found in Indiana and is often confused with giant hogweed (Figure 5). Reactions to both these two plants are dependent on an individual’s sensitivity, but they both require ultraviolet light to cause damage. The compounds found in these plants that cause this reaction are suspected to be used in these plants as protection against UV light because of their ability to absorb UV light9.


Figure 5. Cow parsnip (left) and Giant hogweed (right). The two plants can be easily confused. The identification is the stature and details. (Photo Source: (left) Gary A. Monroe, No. USA, CA, Marin Co., Point Reyes National Seashore, as seen on USDA Plant Database and (right) Terry English, USDA APHIS PPQ, Bugwood.org).
The first indication that you are looking at giant hogweed is the plants sheer stature. Giant hogweed can reach a height of 15 feet tall. Its stems can be up to 4 inches in diameter, and its leaves can be 5 feet broad. Even the inflorescence is large, up to 2.5 feet wide. Cow parsnip can reach up to 8 feet, but are more commonly 4 to 5 feet tall10,11. The stem has a diameter of at most 2 inches and leaves can get up to 2.5 feet broad. Although cow parsnip has lobed leaves, they are not as detailed and deeply loped as giant hogweed’s. The stems of cow parsnip are green or light purple and have fine hairs giving it a fuzzy appearance, but giant hogweed has coarse hairs and purple blotches. These hairs are most noticeable where they circle the stem at the nodes.
Stinging Nettle
Stinging nettle (Urtica dioica L.) is a very common sight in the woods, on the banks of rivers, and in waste areas in Indiana (Figure 6). Touching stinging nettle can produce itching and welts; walking though stinging nettle with shorts or sandals is not advised. Stinging nettle is armed with small hairs that, when touched, can inject a cocktail of histamine, serotonin, acetylcholine and formic acid11 (Figure 6). Histamine causes an immune reaction in the body, serotonin and acetylcholine are neural transmitters and formic acid is the same compound involved in bee stings and fire ant bites.


Figure 6. (Left) Stinging nettle and (right) stinging nettle's hollow hairs. (Photo Source: left, Uwe H. Friese, Bremerhaven 2003, right, Donar Reiskoffer. As seen on Wikipedia.)
Stinging nettle often grows in patches and can become quite tall, growing up to 5 feet, but I generally see it between 2 to 3 feet tall. Stems are unbranched and leaves are opposite, egg shaped and with serrated margins. Stinging nettle can be confused with a couple other plants in Indiana, such as white snakeroot (Ageratina altissima or Eupatorium rugosum), Canadian clearweed (Pilea pumila), and smallspike false nettle (Boehmeria cylindrical). To identify stinging nettle, look carefully at the stems to see if the obvious stinging hairs are present.
Spurges
The plants in North America that belong to the group of plants called the spurges (Euphorbia spp. or Chamaesyce spp.) often have a milky sap that is an acrid latex compound. This sap is a mild skin irritant, but is also poisonous and is considered a carcinogenic12,13,14. Like cow parsnip and giant hogweed above, exposure to the sun induces irritation. There are many plants that belong to this group. They include such notables as poinsettia, prostrate spurge, spotted spurge, and leafy spurge. This is a large group of plants that includes several species, but a common uniting feature is a three chambered ovary leading to a three lobed capsule6 (Figure 7). If you have ever inspected a Christmas poinsettia you would see these capsules nestled in the colorful bracts of the plant.

Figure 6. Three chambered capsule. (Photo Source: Richard A. Casagrande, Univrsity of Rhode Island, Bugwood.org)
References
1. Lerner, B.R. 2001. Poison Ivy. Purdue Cooperative Extension Service. Bulletin HO-218 <http://www.hort.purdue.edu/ext/HO-218.pdf>
2. USDA. 2006. USDA Plant Data Base. <http://plants.usda.gov>
3. Schwartz, L. 1941. A protective Ointment against Ivy Poisoning. The American Journal of Nursing. Vol. 41, 6:675-678
4. Peterson, L.A. 1977. Petersen Field Guides: Edible Wild Plants, Eastern/Central North America. Houghton Mifflin Company, Boston, New York. pp. 182 and 186
5. Sherman, R.B. 1902. Ivy poisoning: with report of a case. The American Journal of Nursing. Vol. 2, 9:660-668
6. Britton, N. and A. Brown. 1913. An Illustrated Flora of the Northern United States and Canada (Amaranth to Polypremum). Dover Publications Inc., New York. Vol. 2
7. Frey, R.G. 1978. Did Socrates Commit Suicide? Philosophy Vol. 53, 203:106-108
8. Sullivan, J. 2001. A note on the death of Socrates. The Classical Quarterly, New Series. Vol. 51, 2:608-610
9. Daniel, O., M.S. Meier, J. Schlatter, P. Frischknecht. 1999. Selected phenolic compounds in cultivated plants: ecologic functions, health implications, and modulation by pesticides. Environmental Health Perspectives. Vol. 107 Suppliment 1 pp. 109-114
10. Nice, G., B. Johnson, and T. Bauman. 2004. The infamous giant hogweed. Purdue University Extension Weed Science. Bulletin WS-32-W <http://www.btny.purdue.edu/weedscience/2004/articles/gianthogweed04.pdf>
11. Michigan Department of Agriculture and the United States Department of Agriculture. Giant Hogweed Heracleum mantegazzianum an attractive but dangerous noxious weed – Have you seen this plant? <http://www.michigan.gov/documents/MDA_Hogweed_Brochure_2_115074_7.pdf>
12. Goetz, R.J., T.N. Jordan, J.W. McCain, and N.Y. Su. Indiana Plants Poisonous to Livestock and Pets: Stinging Nettle Wood (Bull) Nettle. Cooperative Extension Service, Purdue University. <http://vet.purdue.edu/depts/addl/toxic/plant31.htm>
13. Elpel’s, T.J. 2004 Botany in a Day. 5th ed. HOPS Press, LLC
14. Adolf, W., E. Hecker. 1975. On the active principles of the spurge family. Journal of Cancer Research and Clinical Oncology. Vol. 84 3:325-344
Multiple Ears on the Same Shank - (Bob Nielsen)
Last year there was a rash of reports from cornfields throughout Illinois and Indiana of an odd multiple-eared phenomenon that I termed the “bouquet” effect (Nielsen, 2006). The nature of the “bouquet” effect was multiple ear shoots that developed from a single ear shank and, in the worst cases, none of the ears successfully pollinated or set kernels. As I indicated last year, multiple ears on a single plant are not unusual, but the multiple ears usually develop separately from individual stalk nodes.
I suspected then that certain genetic backgrounds were probably more prone to developing multiple ears from the same ear shank. In recent weeks, I’ve noticed a benign form of the multiple-ear characteristic appearing in a hybrid with nearly identical genetics to one that developed dramatic “bouquets” of multiple ears last season.

Fig. 1. Example of two ears developing on single ear shank. The primary ear was in late milk (R3) stage of kernel development.

Fig. 2. Closer view of attachment of second ear to lower node of ear shank.

Fig. 3. Even closer view of attachment of second ear to lower node of ear shank.
The fact that multiple ears sometimes develop from a single ear shank in and of itself is not unusual. Ear shank development essentially replicates the developmental sequence of the main stalk of the plant. The ear shank is comprised of nodes and internodes. Each node develops a leaf like the main stalk, albeit referred to as husk leaves. The ear shank terminates with a reproductive organ (the female ear) somewhat akin to the main stalk terminating with a reproductive organ (the male tassel).

Fig. 4. Comparison of second ear with primary ear.

Fig. 5. Closer view of second ear.
Additional ear shoots can develop from individual nodes of the ear shank like additional ear shoots that develop from individual nodes of the main stalk (Nielsen, 2007a). Normally the ear shank does not initiate these secondary ears or ears initiate but quickly cease development due to apical dominance from the apical ear.
It remains to be seen whether the more dramatic “bouquet” version of multiple ears on a single ear shank will develop this year. One of my observations last year was that the “bouquet” symptom appeared more frequently where kernel set on the main ear was severely restricted (e.g., severe corn rootworm beetle silk clipping).

Fig. 6. Example of two ears developing on single ear shank. Husks removed to show ear shank.
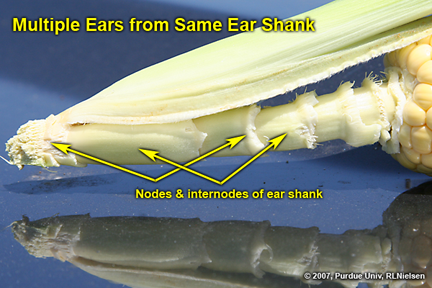
Figure 7. Closer view of ear shank showing nodes and internodes

Fig. 8. View of attachment of second ear to what appears to be the second lowermost node of the ear shank.
Related References
Nielsen, R.L. (Bob). 2006. A Problem with “Bouquets”. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/articles.06/Bouquets-0912.html>. (URL accessed 7/23/07).
Nielsen, R.L. (Bob). 2007a. Ear Size Determination in Corn. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/timeless/EarSize.html>. (URL accessed 7/23/07).
Nielsen, R.L. (Bob). 2007b. Kernel Set Scuttlebutt. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/timeless/KernelSet.html>. (URL accessed 7/23/07).
![]()
Some Options for Supplementing Inadequate Forage Supply – (Keith Johnson and Committee, Indiana’s Grazing Lands Conservation Initiative)
An early spring freeze and extreme dry weather this season has resulted in less than average forage production on many Indiana farms. Because of these weather-related concerns, the Indiana Grazing Lands Conservation Initiative committee is suggesting ways for livestock producers to supplement limited supplies of winter feed.
Not all of the suggested options will work for every producer but perhaps one or a combination of the options suggested will help reduce the shortage of winter feed supplies.
Haying and Grazing of Conservation Reserve Program (CRP) acres may be an option. Contact your county FSA office to see about authorized Emergency or Managed Haying and Grazing of CRP. Also talk with neighboring CRP owners about using their forage through CRP’s authorized haying/grazing provisions. While this feed may not be of high forage quality, supplementing it with a small amount of energy and protein may be adequate for dry stock and pre-lactation animals.
Fallow wheat land can be an excellent location for growing late-summer forage. Some suggestions include seeding spring oat at 2 bushels per acre along with forage turnip at 2 pounds per acre. Seeding can be done from mid-July through very early September. The forage mixture is best harvested as silage or by grazing. Because of the high quality of this feed it is suggested that it be limit fed to livestock with less nutrient requirements at a rate of one-fourth oat and turnip, and three-fourths low quality forage like corn residue. When grazed, the perfect solution would be to have an adjoining field of harvested corn or lower quality CRP forage and to strip graze the oat and turnip combination. Livestock should have access to limited amounts of the oat and turnip so they don’t fail to eat the corn residue. With adequate acres this feed could be used from corn harvest until early spring. An article discussing use of spring oat as a fall and winter feed resource can be found on line at <http://www.agriculture.purdue.edu/AgAnswers/story.asp?storyID=4529>. A publication from the University of Illinois entitled “Extending Fall Grazing with Brassicas and Cereal Grain” is also available on line at <http://www.livestocktrail.uiuc.edu/pasturenet/paperDisplay.cfm?ContentID=8164>.
Other seeding options and considerations. Early fall seeding of annual ryegrass, winter wheat or winter cereal rye can produce late fall, winter and early spring feed. Refer to the labels of herbicides used in the past year or two on the acreage to be seeded to avoid carryover concerns. If this is not an option on your farm consider renting wheat land with adjoining corn residue acreage from a nearby cash grain farmer. It can improve his return per acre as well as provide you with additional low cost feed.
Cereal winter rye, winter wheat or spring oat can be broadcast into standing corn by airplane by early September with the intention of grazing the small grain forage and corn residue together. This seeding option has some risk as adequate soil moisture needs to be available for germination of the broadcasted seed and the successful establishment of the seedlings.
If fencing is a concern for grazing, consider a temporary single high-tensile 12.5 gauge electrified wire with single corner posts and small fiberglass posts to support the wire. Ensure the fencing is adequate to control livestock. Subdivide the grazable area with a poly wire and tread in posts. Consider allotting a one- to three-day supply of feed and then moving the livestock to minimize possible compaction. The fencing can be easily removed once the grazing is completed. If livestock are not familiar with electric fence they should be properly trained by placing an electric wire across a confined area to allow them to test the single-wire system. If water is not available in the field being grazed it will need to be provided on a daily basis. Livestock water needs are less at that time of year because of cooler seasonal temperatures.
If grazing isn’t an option consider harvesting corn stover behind the combine. If possible, remove the spreader-chopper from the back of the combine to make a windrow of shucks and cobs for round baling or chopping. This will be more costly than grazing but much larger acreages can be available for machine harvest. Harvest all the windrows as any left in the field will impact tillage and emergence of the following crop sown.
If applicable, ensure your activities on cropland are consistent with your USDA Program benefits requirements. Planting cover crops and other forages in rotation, implementing a managed grazing system, and/or baling residue under managed conditions should help provide long-term soil health and maintain program eligibility.
Is there easy access to Wet Distillers Grains (WDG) from ethanol processing facilities? Wet Distillers Grains usually test about 70 percent moisture and can be ensiled with corn residue to make a good source of feed for dry stock and pregnant animals. Mix at a level to keep the moisture content of the mixture over 50 percent so adequate ensiling occurs.
Adding WDG to corn silage can also extend the amount of feed available. WDG can also be fed directly to livestock as a supplemental feed. The WDG do not store very long in open air so you need lots of animals or very little WDG at a time. Knowing the sulfur content of the WDG is important as high levels of sulfur in a ration impacts the well being of the livestock being fed.
Both wet and dry distillers grains can be fed in limited quantities to livestock. Consult with a trained animal nutritionist for proper utilization of these products.
Stockpiling pasture growth for late-autumn use. If adequate rainfall begins soon, there is still opportunity to stockpile cool-season pasture growth in the late summer and early fall. Stockpiling refers to growth of forage in some paddocks of a rotational grazing system in the late summer and early fall and defers the grazing of the forage until mid-to-late fall. The addition of 50 lbs. of N per acre by mid-August in grass-dominant paddocks to be stockpiled should be considered to produce greater forage yield.
Drought-damaged corn harvested as silage. Another forage resource that should be considered is corn that was damaged by dry weather and will not produce an economic grain yield. The whole plant can be harvested as silage and fed as a component of the livestock ration. Contact the Farm Service Agency and crop insurance personnel to see what requirements need to be considered before silage harvest begins. Nitrate level in drought-damaged corn is an issue but nitrate remaining in the forage is reduced after ensiling. Analysis of the silage for nitrate level by a feed testing laboratory will help to determine the upper limit that silage that can be fed so nitrate toxicity will not occur.
Contact your Purdue Extension Educator for additional suggestions on extending the supply of winter feed. The Indiana Grazing Lands Conservation Initiative committee is planning an early winter tour around the state to share examples of some of these feeding options. Watch for announcements for these upcoming events.
Indiana Pesticide Clean Sweep Project – (Kevin Neal, Indiana State Chemist Office)
WHAT: The Indiana Pesticide Clean Sweep Project is designed to collect and dispose of suspended, canceled, banned, unuseable, opened, unopened, or just unwanted pesticides (weed killers, insecticides, rodenticides, fungicides, miticides, etc.) is is being sponsord by the Office of Indiana State Chemist (OISC). This disposal service is free of charge up to 250 pounds pr participant. Over 250 pounds there will be a $2.00 per pound charge. This is a great opportunity for you to legally dispose of unwanted products at little or no cost.
WHO: All public and private schools, golf courses, nurseries, cities, town, municipalities and county units of government or others receiving this notice are eligible to participate.
HOW: Complete the enclosed Pesticide Clean Sweep Planning Form to the best of your ability <http://extension.purdue.edu/pestcrop/2007/issue18/pesticide.pdf>. Fax or email the completed form to Kevin Neal at 765-494-4331 or nealk@purdue.edu no later than Wed., August 1, 2007. Then bring your labeled, leak free and safe to transport containers to the collection site. DO NOT mix materials. In case of an emergency, you should bring with you a list of products you are carrying and a contact phone number.
WHEN/WHERE:
• August 7, 2007: Daviess County Fairgrounds in Elnora, IN
• August 9, 2007: Tippecanoe County Fairgrounds in
Lafayette, IN
• August 14, 2007: Whitley County Fairgrounds in Columbia
City, IN
•August 16, 2007: Decatur County Fairgrounds in
Greensburg, IN
*NOTE: OISC reserves the right to cancel this Pesticide Clean Sweep Project if there is not adequate demand. Participants submitting the enclosed planning form by Aug. 1, 2007 will be contacted immediately if cancellation is necessary.
If you have further questions please contact the Indiana State Chemist office at (765) 494-1492.
![]()