Pest & Crop Newsletter, Entomology Extension, Purdue University
- Refuge Planting Strategies for Bt Rootworm Corn: Geometry is Important
- Winter Temperatures, Corn Flea Beetle Survival, and Potential for Stewart’s Wilt
Refuge Planting Strategies for Bt Rootworm Corn: Geometry is Important – (Christian Krupke, John Obermeyer, and Larry Bledsoe)
- Refuge is required when planting any Bt corn.
- Not all refuges function in the same way.
- Consider rootworm beetle movement when deciding upon refuge configuration.
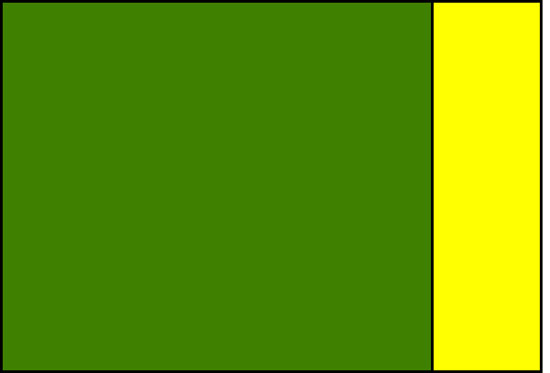
OPTION 1: Block refuge
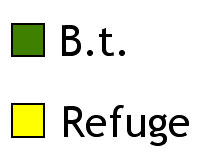
OPTION 2: Split planter (strips)
Despite the recent snowfall, winter has ended and planting time will soon be upon us. For those producers planning on using Bt corn (Bt-RW) to protect their crop from corn rootworms, this means that planting a non-Bt refuge must also be part of the plan. Although we have had access to Bt corn for control of corn borer and other caterpillars for several years, Bt-RW corn for rootworm control has been available for only 2 seasons. Sales of these products have been strong, and should continue to be in 2006. The discussion that follows includes some information to keep in mind when you plant your mandatory refuge for Bt-RW corn– biological factors such as beetle movement and mating that may influence the decision on how to plant a refuge.
Pictured are two diagrammatic representations of common refuge structures. Both meet the refuge requirements of 20% of acreage being planted to non-Bt corn within the same field. Bear in mind that these refuges should be protected from rootworms in some other way (e.g., soil insecticides).
Although planting the refuge is sometimes inconvenient, there is a good reason behind it – resistance prevention. Rememer although Bt-RW corn does provide excellent protection from rootworm larvae, there are individuals that survive exposure and make it to adulthood. If we assume that these individuals have some advantage that enables them to survive Bt-RW corn exposure, and we assume that the advantage is in their genes (heritable), we definitely do not want them mating with each other and passing those genes on to their offspring. The way we try to prevent this from happening is by increasing the odds that these survivors will mate with non-Bt exposed beetles – that is, individuals emerging from the refuge areas. More beetles will emerge from refuge plants than from Bt-RW plants, theoretically flooding the area with “susceptible” genes.

The light colored female western corn rootworm beetle is being mated shortly after emerging from the soil.
One area of research within the Purdue Field Crops program examines the emergence and movement trends of beetles within and between Bt-RW fields. Though this work is ongoing, our results so far indicate that in a 50-acre field, the split planter refuge type (Option 2 above) supplies twice as many beetles to the Bt-RW area overall compared with a block refuge type (Option 1). Remember that in this experiment, large numbers of beetles are a good thing from a resistance management standpoint – we are getting adult beetles where we want them (into the Bt-RW corn) where we hope they will mate with the Bt-RW survivors and dilute those potential resistance genes. This result is not surprising, when you consider the distance that adult refuge beetles in Option 1 must travel to get to the center of the field. This supports the findings of rootworm researchers Dr. Joe Spencer and Dr. Eli Levine at the University of Illinois, indicating that rootworms move a maximum of about 15-20 feet a day. At this rate, getting from the refuge to the middle of a large block of Bt-RW corn would take a beetle several weeks (assuming straight-line travel) – a long time in the life of a rootworm, especially considering that female beetles mate relatively soon after emergence. Clearly, proximity to the refuge is an important factor in refuge design.
The purpose of this is to emphasize that while we have not seen resistance to Bt-RW corn in rootworm beetles, the central premise of a refuge is keeping it that way. However, not all refuge designs are equally efficient in moving and mixing beetles throughout the field. Planting a refuge that mixes both Bt-RW and refuge beetle populations thoroughly (split planter) appears to be the best option for accomplishing this goal, given our current knowledge and options. We are continuing to investigate the effects of refuge construction on beetle movement and mating, so look for more updates on this important research area in the future.
![]()
Winter Temperatures, Corn Flea Beetle Survival, and Potential for Stewart’s Wilt - (John Obermeyer, Christian Krupke, and Larry Bledsoe)
- Corn flea beetle winter survival is expected to be low in northern Indiana.
- Moderate survival is expected for central regions of Indiana.
- Southern counties of the state may have high survival.
- Snow cover in December may have benefited some overwintering beetles.
- Corn flea beetle is a vector of Stewart's wilt of corn, which has two disease phases.
- Management guidelines for low and high susceptible corn is given below.
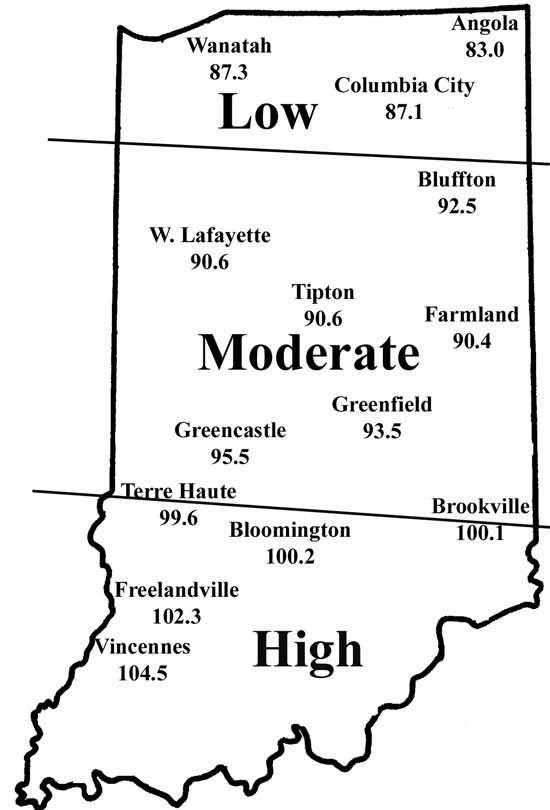
Corn flea beetle is a sporadic pest in Indiana. Winter temperatures in regions where beetles were abundant last season will determine if there is cause to be concerned this season. This is especially important since this insect can transmit the bacteria that cause Stewart’s disease in corn. The severity of the disease correlates well with winter temperatures because the bacterium survives in the gut of the overwintering beetles. Warmer temperatures result in higher beetle survival, and greater potential for Stewart’s disease. To determine the potential severity of Stewart’s disease, add the average daily temperatures for the months of December, January, and February. If the sum is below 90, the potential for disease problems to develop is low. If between 90 and 100, moderate disease activity is a possibility. Sums above 100 indicate a high probability that severe problems will develop for susceptible corn. To help you better gauge the potential for corn flea beetle activity in your area, and thus the potential severity of the threat of the disease, we have created the following state map. According to the temperature model, there is low probability of corn flea beetle activity and subsequent disease in northern Indiana, moderate activity in central counties. In most of southern Indiana, conditions were very favorable for beetle survival that may result in the appearance of Stewarts’s wilt in sensitive hybrids/inbreds this spring.
This temperature model for corn flea beetle has been around many years and has been fairly accurate in predicting the activity of this pest the following spring. However one inherent flaw is that the model is based on ambient air temperatures, not temperatures under leaf litter and grass clumps where this pest overwinters. As well, snow cover, which can provide an excellent insulating blanket for the insect, may protect some beetles from winterkill. Even with this “disclaimer” statement, we think the 2005/2006 winter was cold enough to have negatively impacted overwintering beetles in northern Indiana. Also, flea beetle numbers have been low statewide, in general, for the last couple years.
There are two phases of Stewart’s disease: a wilt phase and a leaf blight phase. In the wilt phase, plants wilt rapidly, usually at an early stage of growth. Leaves emerging from the whorl of infected plants are often the first to wilt. Internal tissues at the growing point are discolored or hollowed out. Faint green to yellow streaks containing corn flea beetle feeding marks are visible on one or more leaves. If stalks of wilted plants are cut, it may be possible to see yellow, moist beads of bacterial ooze. Sweet corn hybrids are especially susceptible. Some dent corn inbreds, and occasional dent corn hybrids, and some popcorn lines are susceptible as well. Dent corn hybrids rarely wilt after growth stage V5. The leaf blight phase can occur at any time during the growing season, but often does not appear until after tasseling. Lesions are long and narrow, with pale green to yellow streaks and irregular or wavy-margins. Streaked areas die and become straw-colored. Severely infected leaves may die prematurely. Lesions on leaves of older plants may be confused with northern corn leaf blight. It is usually possible to see beetle feeding tracks in Stewart’s disease lesions.
Management decisions made now, should be based on the corn’s susceptibility to the disease and anticipated risk. Low susceptibility/risk - pest managers should scout fields and apply a foliar rescue treatment after emergence if (1) beetle feeding damage becomes severe, (2) there are 5 or more beetles per plant, and (3) seedlings are growing slowly (e.g., cool temperatures). High susceptibility/risk - sample field edges and in-field areas of grass weed residue (i.e., overwintering sites) before planting to assess overwintering beetle survival and potential beetle movement to emerging corn plants. A sweep net is an ideal sampling tool for this pest. If any beetles are discovered at this time, an at-planting insecticide application is warranted. Cruiser and Poncho insecticide treated seed are systemic insecticides that should give good control of flea beetle in the early seedling stage. Cruiser and Poncho must be applied to seed by commercial seed treaters. The low rates of the seed treatments are expected to provide protection from emergence to 2-leaf corn, whereas the higher (rootworm) rate should protect corn through the 5th leaf stage. If insecticide treated seed is not an option, foliar insecticides broadcasted at the time when corn spikes should provide 7 to 10 days of residual protection from beetle feeding. CAUTION: treating of field edges and waterways for beetle control may be an off label application. Avoid movement of insecticides, including soil-bound materials into aquatic environments.
![]()
Broadleaf Weed Control in Winter Wheat – (Bill Johnson and Glenn Nice)
Unlike just a few years ago when there were only a handful of herbicides registered for the control of broadleaf weeds in winter wheat grown in Indiana, there are now a number of herbicides available to control weeds in wheat. The most common broadleaf or perennial weed problems we run into at this time of year in Indiana wheat include chickweed, deadnettle, henbit, dandelion, mustards, field pennycress, shephardspurse, Canada thistle, and wild garlic. Some of the commonly used herbicides, rates, their application timings, and weeds controlled are listed in the table below.
Click to see Table
Herbicides to control broadleaf weeds in winter wheat.
It is also important to be aware that restrictions exist concerning application timing of these herbicides to avoid crop injury. Phenoxy herbicides, such as 2,4-D and MCPA, control a number of annual broadleaf weeds and are the least expensive of these herbicides to use. However, proper application timing of the growth-regulating herbicides 2,4-D, MCPA and Banvel is critical to avoid crop injury and possible yield losses. These herbicides can cause substantial crop injury and yield loss in small grains if applied before tillering begins or after development of the grain heads has been initiated.
The exact time at which grain heads have been initiated is not easy to determine, but this event always just precedes stem elongation. The occurrence of stem elongation can be easily detected by the appearance of the first node or “joint” above the soil surface, commonly referred to as the “jointing stage.” Pinch a wheat plant stem at the base between the thumb and forefinger and slide your fingers up the stem. The presence of a node or joint will be felt as a hard bump about an inch above the soil surface. Slicing the stem lengthwise with a sharp knife will reveal a cross section of the hollow stem and solid node. If jointing has occurred, applications of 2,4-D, MCPA and Banvel should be avoided because crop injury and yield loss are likely. Research from the University of Missouri Weed Science program has shown a 3 to 6 bushel per acre yield loss from 2,4-D and Banvel applications to wheat after the jointing stage.
MCPA alone at labeled rates should be applied before jointing. However, the amount of MCPA applied in Bronate, a combination of bromoxynil and MCPA, is low enough to permit later applications.
As a final note, many wheat fields in Indiana contain wild garlic and wild onion. Although not considered as strong competitors with a wheat crop, wild garlic (Allium vineale) and wild onion (Allium canadense) are both responsible for imparting a strong odor to beef and dairy products. Wheat producers and grain elevator operators are very familiar with dockages that occur with the presence of wild garlic or onion bulbs in their harvested grain. Found throughout Missouri, wild garlic is a native of Europe, while wild onion is native. Despite the fact that these perennials both occur in similar habitats, wild garlic occupies the majority of small grain settings, including wheat.
Control measures for wild onion and wild garlic will differ. Producers, consultants and industry personnel will want to make certain that they are able to distinguish between these two weed species. The vegetative leaves of wild garlic are linear, smooth, round and hollow (flowering stems are solid). A major difference with wild onion is that its leaves are flat in cross section and not hollow. Another varying feature are the underground bulbs. Wild garlic’s bulbs have a thin membranous outer coating while wild onion’s bulbs have a fibrous, net-veined coating.
Harmony Extra (thifensulfuron + tribenuron) is the herbicide most commonly used for control of garlic in wheat, plus it controls a relatively wide spectrum of other broadleaf weeds and possesses a fairly wide application window. Harmony GT (thifensulfuron) also has activity on wild garlic, but is considered to be slightly weaker than Harmony Extra. Peak is also labeled and effective on wild garlic in wheat, but it is fairly persistent in soil. The Peak label does not allow one to plant double crop soybean following wheat harvest in Indiana. Wild onion is controlled with 2,4-D. Keep in mind that both of these weeds are perennials and the full labeled rate is needed for adequate control.
Over the last couple of years, dandelion infestations in wheat have increased dramatically, particularly in the eastern part of Indiana. The best dandelion control is usually obtained with fall applications of glyphosate before wheat is planted. So keep this in mind for fields that will be planted to wheat in coming fall. For this spring, the best approach to dandelion management in wheat will be the higher rates of 2,4-D, Stinger, or Curtail. Stinger will have the widest application window and can be applied up until the boot stage.
![]()

The 2006 Weed Control Guide for Ohio and Indiana – (Glenn Nice, Bill Johnson, and Tom Bauman)
The 2006 Weed Control Guide for Ohio and Indiana contains 189 pages of herbicide and weed management information. The weed guide has herbicide efficacy ratings; lists labeled rates and restriction comments; has several tables including rotation and grazing restrictions; herbicide family and mode of action information; and a section for control of specific problematic weeds.
The weed guide can be ordered for $7.50 by calling (614) 292-1868 or by contacting your Purdue Extension Educator. It is also available on line at http://www.btny.purdue.edu/Pubs/WS/WS-16/
New to 2006 is a section on popcorn weed control. This 15 page section is tailored for popcorn producers and focuses on the herbicides labeled for popcorn. It includes a weed response table and some information regarding herbicide use restrictions for specific products.
Herbicides included in the 2006 popcorn section:
Atrazine |
Alachlor + Atrazine |
Lexar |
Acetochlor |
Callisto |
S-metolachlor |
Acetochlor + atrazine |
Guardsman Max |
S-metolachlor + atrazine |
Alachlor |
Lumax |
Outlook |
Prowl |
Pendimax |
Aim EW |
Basagran |
Bromoxynil |
Laddok S-12 |
Accent SP |
Beacon |
Callisto |
Dicamba |
Dicamba + atrazine |
Distinct |
Exceed |
NorthStar |
Permit |
Priority |
Spirit |
Some herbicides have been removed from the guide and others have been added. Canopy XL® (Authority® [sulfentrazone] + Classic® [chlorimuron]) has been removed from the soybean section of the guide. DuPont has re-released the old Canopy® (Sencor® [metribuzin] + Classic®), it was announced too late to make it into this years weed guide. Weeds controlled with Canopy® will be similar to Canopy XL® with a few exceptions. Canopy® will provide better control of ALS-resistant ragweeds, than Canopy XL®. Canopy EX® (Express® [tribenuron] + Classic®) has been added to both the burndown and soybean sections.
Epic® (Define [flufenacet] + Balance Pro [isoxaflutole]) has been dropped from the corn soil applied section and replaced with Radius®. They both are premixes of Define® and Balance Pro®. Radius has a reduced concentration of active ingredients and is a suspension concentrate formulation. Epic® has 48% flufenacet and 10% isoxaflutole per pound of product and Radius® has 35.71% flufenacet and 4.29% isoxaflutole per gallon of product.
Two other new trade names that you will find in the 2006 Weed Control Guide are Targa® (Assure II® [quizalifop]) in the soybean weed response table and Sandea® (Permit® [halosulfuron]) in the corn postemergence section.
In the grass pasture section Ally® has been completely replaced by Cimmaron®. Both products contain 60% metsulfuron-methyl.
If you are interested in other tools brought to you by the Botany & Plant Pathology Weed Science Team see the flyer and ordering information at this web address http://www.btny.purdue.edu/weedscience/2006/WeedTools06.pdf.
![]()
Nitrogen Accumulation by Annual Grass Weeds in Roundup Ready Corn Production – (Bill Johnson)
It is anticipated that adoption of Roundup Ready corn will proceed at a fairly rapid pace over the next couple of years in the eastern cornbelt. As growers adopt this technology, we anticipate that they will also begin shifting their weed control programs from those that rely heavily on soil-applied acetamide-atrazine premixes to those that are less reliant on the premixes and more reliant on postemergence glyphosate in Roundup Ready corn or glufosinate (Liberty) in Liberty Link corn. So, if adoption of Roundup Ready corn and changes in herbicide use patterns proceed as expected, we will be going from a system which was largely devoid of early-season weed pressure to a system where early-season weed infestations will be common and require broadspectrum postemergence herbicides for effective control and protection against yield loss. In addition, the current high prices of nitrogen fertilizer will also cause some growers to consider reducing nitrogen rates to cut costs of production.
It is important to understand that weeds are just like crop plants and will utilize soil nutrients in a similar manner to grow and reproduce. What is not well understood is how much nitrogen is utilized by weeds and the effect of nitrogen use by weeds will have on crop yields. Over the past 7 years, I have had a couple of my graduate students conduct research projects that involved nitrogen accumulation by weeds in corn and impact on corn yield. In the next couple of articles I will summarize the results of these projects.
The first project I will discuss was an evaluation of annual grass weed interference and nitrogen accumulation in no-till, Roundup Ready corn. The objective was to determine the interactive effects of grass weed interference and sid-dressed N applications on corn and weed growth and N content and corn yield. The experiment was conducted in 1999 and again in 2000 on a silt loam soil with 2.5% organic matter. The experimental area was a no-till site. Soil-applied broadleaf herbicides were applied to control broadleaf weeds and allow grass weeds to emerge with the corn. The grass weeds present in this study consisted of giant foxtail, barnyardgrass, and large crabgrass and a combined density of approximately 30 plants per square foot. Ammonium nitrate fertilizer was surface applied at 100 lb N/A just prior to planting. We utilized a relatively low or “threshold” rate of nitrogen in an attempt to tease out the effects of N accumulation by grass weeds on corn growth and yield.
The grass weeds emerged at about the same time or slightly later than the corn and were controlled with glyphosate when they were either 3, 6, 9, or 12 inches tall. After the grass weeds were controlled at the specific timings, the plots were kept weed-free for the remainder of the growing season. To determine if side-dress nitrogen could be utilized to overcome the effects of early-season grass weed competition, the weed removal timing treatments were duplicated and an additional 40 lbs of N/A was applied to those plots when corn was 2 feet tall. Corn and grass weed tissue samples and soil samples (2 feet deep) were collected from weedy and weed-free plots at each grass control timing and at corn harvest. Plant samples were analyzed for total Kjeldahl N and soil samples analyzed for nitrate and ammonium content. The experimental design was a randomized complete block with four replications each year.
The results showed that grass weeds accumulate quite significant amounts of nitrogen on a per area basis. At the 3 inch removal timing, grass weeds contained similar amounts of N on a per area basis as corn. By the time grass weeds are 12 inches tall, they had 50 to 63 lbs of N/A in 1999 and 16 to 32 lbs of N/A in 2000. This amounts to about 3 times as much N as contained in corn biomass in 1999 when the grass weeds emerged with the corn, and about ½ as much N in corn biomass in 2000, when corn emerged about 10 days before the weeds emerged.
The main effect of weed removal height on corn yield was similar in both years. Corn yield and N content of corn biomass were similar to the weed-free controls with grass interference up to 6 inches in height before control measures were implemented. Yields were lower in treatments with grass weed interference until 9 inches or greater in height and there was less N in the corn biomass than the weed-free controls. Side-dress N had a positive effect on recovery of corn yield due to weed interference in 2000 when adequate late season precipitation was available, but had no effect on corn yield in 1999 when late-season precipitation was limited.
In summary, when grass weeds at a density of 30 plants per square foot emerge at the same time as corn, they should be controlled before reaching 6 inches in height to avoid excess N accumulation and crop yield loss. Surface-applied ammonium nitrate as a side-dress treatment was effective in overcoming the competitive effects of early-season weed interference in corn in a year with adequate late-season precipitation, but was not effective in a dry year. The best opportunity for utilizing side-dress N to recover yield due to weed interference will be to inject the N into the soil after postemergence weed control measures are conducted to minimize the amount tied up by microbes as they decompose the weed biomass on the soil surface.
Reference:
Hellwig, K. B., W. G. Johnson, and P. C. Scharf. 2002. Grass weed interference and nitrogen accumulation in no-tillage corn (Zea mays L.). Weed Sci. 50:757-762.
Asian Soybean Rust - (Gregory Shaner)
- Will this desease be a threat this year?
Last year growers throughout the Midwest were concerned about the threat posed by Asian soybean rust, caused by a fungus that was first detected in the continental U.S. in November of 2004. However, rust spread very slowly last summer, and was confined to the southeastern U.S. Even there, rust really didn’t spread much until after mid September, by which time the risk to soybean in the Midwest was low.
What can we expect this year? No one can say what the risk will be in the Midwest during 2006. During the winter of 2004-05, rust evidently only survived the winter in central Florida. From there, spores needed to move west into the Mississippi Valley in order to establish a source of inoculum that could move up into Ohio, Indiana, and Illinois. Westward movement was very slow last year.
This winter, plant pathologists in the South have been scouting kudzu in Florida and along the Gulf Coast. They found rust at 21 sites: 11 in Florida, 4 in Georgia, 5 in Alabama, and 1 in Texas (the Texas finding was in a late-maturing soybean field). Even in areas where most kudzu was defoliated by frost, rust was found in protected sites where plants had kept their leaves. Given the few people who are scouting, there were doubtless other sites where rust overwintered. It is likely that the discovered sites are only a fraction of the total number.
Over the past couple of weeks, kudzu north of the freeze line has been sending out new leaves, and plant pathologists are now scouting these plants to track the northward movement of rust. The rate at which rust moves northward this spring may provide one clue as to our risk for rust in Indiana. Weather will also have a big effect. Wind patterns will determine whether rust spores in southern areas will be carried up here. Local weather will affect the ability of these spores to infect soybean if they do reach Indiana. Weather throughout the summer will determine how rapidly rust builds up once it is established in local fields.
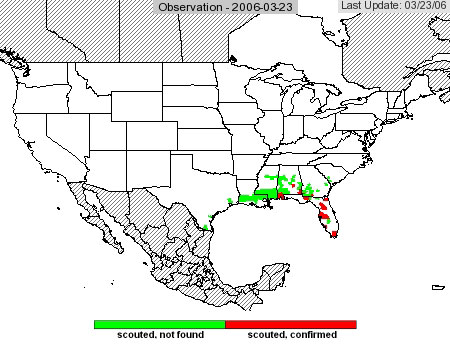
The USDA soybean rust web site that was available last year is still running and will operate throughout the season. Access it at http://www.usda.gov/soybeanrust/. At the lower left of the home page, under Spotlights, there is a map of the U.S. Clicking on the text just to the right of this map will bring up a large rust observation map. Right now, there are green or red dots in the southern U.S. These are counties where rust is present (red) or where someone has looked for rust but didn’t find any (green). A calendar at the left side of page allows the user to scroll back in time. For example, clicking on March 1 will bring up a new map that shows a red county in south Texas. This was a field of soybean that had rust, but after the plants were harvested rust was presumably eliminated, so this county is presented as green in the current map.
As the season progresses and scouting intensifies, more counties will appear as green on the map. As rust spreads, there will be more red counties. This map is an excellent way to track the progress of rust. From this map, users can access maps and commentaries for each state, where they can find control recommendations and other information.
![]()
Yellowing of Wheat - (Gregory Shaner)
- It's the time of year when wheat fields may show a yellow mosaic.
Each year I alert readers to symptoms caused by two soilborne virus diseases of wheat: soilborne wheat mosaic and wheat spindle streak mosaic. The viruses responsible for these mosaic diseases—Soilborne wheat mosaic virus and Wheat spindle streak mosaic virus—cause a yellowing of foliage. Soilborne wheat mosaic virus infection results in development of narrow, pale green to yellow, wavy-margined streaks on the leaf blade. Symptoms caused by Wheat spindle streak mosaic virus infection are similar, but the streaks tend to taper at both ends, hence the name “spindle.” From a distance, fields or parts of fields are pale green or yellow, as though they are deficient in nitrogen.

Differences in resistance to Wheat spindle streak mosaic virus and Soilborne wheat mosaic virus. The varieties in the lower left and upper right are highly resistant. The three varieties in starting at the right-hand side of the front row are susceptible. They are pale green and stunted. This degree of damage early in the season would probably result in some loss of yield.

Servere mosaic on a susceptible variety of wheat.
Either virus, or both, could produce these symptoms.
The viruses persist in a common soilborne fungus, Polymyxa graminis. This fungus infects wheat roots in the fall. Infection by the fungus itself is of little consequence, but it does allow transmission of the viruses to the plant. Wetter areas of fields may show more intense symptoms because the fungus prefers moist conditions. Rains that fell during late September and again during late October may have been sufficient for infection in some fields. Although infection occurs in the autumn, symptoms of virus infection don’t appear until the following spring. The timing of symptom development depends on weather. Intermittent periods of warm and cold weather favor symptom development. The recent warm weather followed by the current cold weather (March 21) may trigger symptom development as temperatures rise again.
It is very difficult to distinguish these two diseases based on symptoms. Both viruses may be found in the same field, and both viruses may infect a single plant. Wheat spindle streak virus tends to be more uniformly distributed throughout fields than is Soilborne wheat mosaic virus.
Most varieties of soft red winter wheat grown in Indiana have some degree of resistance to these viruses. They may show some yellowing during periods of fluctuating temperatures during the spring, but once the cold weather is past, these varieties tend to outgrow the symptoms on lower leaves and there is probably little damage. A few varieties are more susceptible. The intensity of yellowing is greater, and is accompanied by stunting, reduced tillering, and death of some plants in the field. These varieties will suffer economic damage from these diseases. Some varieties show a rosette symptom when infected by Soilborne wheat mosaic virus. They produce numerous, stunted tillers. There is no remedial action that can be taken at this stage. If a variety develops severe symptoms, don’t plant it again next year. There are plenty of varieties with good resistance.
![]()
Identifying Wheat Growth Stages - (Gregory Shaner, Shawn P. Conley, and Bill Johnson)
For effective management of wheat, it is important to recognize the stages of growth as the crop develops. Heading date is a common indicator of relative maturity, but the ability to recognize other growth stages is important for judging the progress of the crop and making management decisions, such as application of fertilizer, herbicides, or fungicides, and for predicting the consequences of disease or injury to the crop. The Feekes and Decimal (Zadoks) scales are the most common growth stage systems for wheat. The Feekes scale is older and has been used widely since the early 1950s. The Decimal scale is designed to make finer distinctions among stages of crop growth, and is probably used more in Europe than in the U.S., although pesticide labels in the U.S. are starting to use both scales.
The Feekes scale divides growth stages into 11 major categories. Head emergence, flowering, and grain filling (Feekes Growth Stages 10 and 11) are further subdivided. The Feekes Growth Stage scale is presented on the following Table 1, with a description of the crop development stage that corresponds to each number. The Decimal scale comprises 9 major divisions (1-9), with 10 possible subdivisions (0-9) for each major division. For example, the tillering stage is denoted by 2 in the Decimal scale, and the second digit indicates the number of tillers per plant. The Feekes scale simply notes whether tillers have begun forming (FGS 2), or whether tillering is essentially complete (FGS 3), without requiring the counting of tillers, although tiller number per plant could be appended after the “2”, e.g. FGS 2.4.
The most difficult task in describing crop growth stage is determining leaf number and tiller number. Accurate determination of leaf and tiller number requires that plants be dug up and carefully separated. To determine leaf number, position the plant so that the first true leaf is on the left. Because winter wheat has an opposite leaf arrangement the next leaf will be on the right side of the plant. By spring, the first 2 leaves may have died and withered, so the plant needs to be inspected carefully to find the remnants of these leaves. The next leaf would be counted only if that leaf was at least one-half the length of the preceding leaf. Continue counting leaves up the stem until the total number of leaves is determined. It is important that tillers be differentiated from leaves and counted separately. To distinguish tillers from a leaf look for the presence of an independent sheath, called a prophyll, which is located at the base of each tiller. Unlike leaves, tillers are counted as soon as they emerge. Once leaf number and tiller number have been identified, the subsequent key characteristics to be noted are node formation, flag leaf emergence, boot stage, head emergence, flowering, and finally grain development.
In winter wheat, the period from beginning of tillering to completion of tillering may extend for a considerable time, from autumn into the following spring. Likewise, the precise limits of FGS 4 and 5 are not clear. Depending on planting date, variety, and weather in the fall, plants may reach the pseudo stem erection stage in the fall, or only in the spring as the crop comes out of dormancy.
Jointing (FGS 6, DC 31) can be clearly determined. The original Feekes scale simply defined stage 6 as when the first node was visible at the base of the shoot. The Decimal scale provides a more precise definition for this stage, namely when the distance between the crown and the first stem node is at least 1 cm (0.4 in.), and we have included this in the growth stage table. When the second aboveground node is at least 2 cm (0.8 in.) above the first node, the plant has reached FGS 7 or DC 2. The ability to recognize FGS 6 is important because it’s the cutoff for many herbicides, especially those that contain 2,4-D, dicamba (Banvel, Clarity), or MCPA. Application of these products after jointing can result in malformed heads, sterility, and reduced yield.
Once the flag leaf blade has fully emerged, the flag leaf sheath extends. By this time, the head enclosed in this leaf sheath is swelling, and the plant enters the boot stage (FGS 10). The heads of all plants in a field will not emerge from the boot synchronously. Stages 10.1 through 10.5 are best assigned according to when heads on about half the plants have reached the indicated degree of emergence.
Flowering in wheat begins roughly in the middle of the head and progresses both upward and downward. Flowering at a given position in the head can be judged by the presence of extruded anthers.
Ripening is judged by removing developing kernels from the center of several heads and determining whether the contents are watery, milky, or at the soft or hard dough stages.
By the time wheat has reached FGS 8, leaves F-5 and below are usually withered, from infection by Septoria, Stagonospora, and other fungi. The next leaf up (F-4) usually withers about the time heads have fully emerged. In the absence of Septoria and Stagonospora blotches, powdery mildew, or other foliar diseases, leaves F-3 through F should remain green until the wheat approaches maturity. Often, however, disease destroys leaves at each layer of the canopy prematurely. Fungicide control is aimed at maintaining these leaves, particularly F and F-1, in a healthy condition.
If a grower is planning to apply a fungicide at flag leaf emergence (FGS 8), it would be helpful to know when that stage will be reached, relative to some earlier, easily determined growth stage. The jointing (FGS 6) and 2-node (FGS 7) stages can be accurately determined if a wheat field is monitored frequently. The time required for a plant to progress from either of these stages to FGS 8 is not constant. It depends on weather conditions, particularly temperature. Over many years, we have monitored wheat crop development in various field trials, and the following observations can give some guidelines for the time required for plants to progress from one growth stage to another. We found that it takes about a week to progress from FGS 6 to FGS 7, and another 8 days to go from FGS 7 to FGS 8 (with a range of 5 to 10 days). It can take from 3 to 8 days for the flag leaf blade to fully expand (going from FGS 8 to FGS 9). It can take from 9 to 16 days to progress from FGS 9 to full head emergence (FGS 10.5) or the beginning of flowering (FGS 10.5.1).
To correctly determine crop growth stage, identify the following characteristics in order. Refer to Table 1 for the corresponding numerical assignment.
- Count the leaves on the main shoot
- Count the tillers
- Count the nodes
- Flag leaf emergence
- Boot stage initiated
- Head emergence
- Flowering or anthesis
- Grain developmental stage
Click here for Table 1.
Soft Red Winter Wheat Crop Growth Stages
![]()
2006 Popcorn Agri-Chemical Handbook Available Online – (Genny Bertalmio, The Popcorn Institute)
The 2006 Popcorn Agri-Chemical Handbook is now available at http://www.popcorn.org/handbook to insure everyone in the popcorn industry is informed about products registered for use on popcorn or in popcorn storage facilities. The handbook lists agri-chemicals registered, special use restrictions, the status of a chemical under special review by the Environmental Protection Agency (EPA), residue tolerances established by EPA and CODEX tolerances.
The Popcorn Board urges you to provide the above link to your growers or download, print and distribute the updated version of this critical information to them. Contact Genny Bertalmio, 312-673-4883 or gbertalmio@smithbucklin.com, for further information or if you require a hard copy.
The Popcorn Board accepts voluntary contributions to insure continued funding of its efforts to provide this important information to the popcorn industry. Checks should be mailed to the Popcorn Board, 401 N. Michigan Avenue, Chicago, IL 60611-4267.

