Pest & Crop Newsletter, Entomology Extension, Purdue University
Soybean Aphid Threshold Clarification- (John Obermeyer, Christian Krupke, and Larry Bledsoe)
- Treatment threshold of ≥250 aphids/plant INCLUDES a week to get field sprayed.
Pest managers in northern Indiana are aware of the soybean aphid situation. Many fields have been scouted and treated when aphid numbers surpassed the threshold of ≥250 aphids/plant, see last week’s Pest&Crop for more specifics. There still exists some confusion about the lag-time from scouting to getting a field treated.
The ≥250 aphids/plant threshold through the R4 growth stages allows for a one week (7 day) window in which to get the field treated without economic loss. In other words, the threshold allows for the fact that aphid populations will be increasing over the period between when populations reach threshold and when the actual treatment is applied. Happy scouting!
![]()
Mexican Bean Beetle Appearing in Southern County Soybean Fields – (John Obermeyer, Christian Krupke, and Larry Bledsoe)
- This pest can rapidly defoliate soybean leaves.
- Pest biology and damage symptoms are given.
- Treatment decisions are based on several variables.

Mexican bean beetle

Larva and characteristic "lacy" defoliation

Mexican bean beetle pupa
While conducting soybean sweeps for the western corn rootworm variant, adult Mexican bean beetle and their damage are quite evident in some fields south of Interstate-70. With the numbers of pupae and adults observed, it is obvious that soon the larvae will be present for another generation. Larvae in high numbers can quickly defoliate soybean, so much that the fields appear frosted almost overnight.
The Mexican bean beetle is actually a cousin of the ladybird beetle, one of the few destructive species of this primarily beneficial family of insects. The adult is oval shaped and copper colored, with 16 black spots on its back. It is about 5/16 inch long and 1/4 inch wide. Females lay yellow, oval-shaped eggs in clusters on the underside of bean leaves. From these eggs, hatch yellow larvae with branched spines that cover their soft bodies. There are 4 larval stages, the final one reaching a length of 1/3 inch, before transforming into a bright yellow pupae. The pupae are usually found attached to the underside of leaves.
Soybean plants can be severely defoliated by both the adult and larval forms of the Mexican bean beetle, though typically, the larvae are more damaging. Larvae strip away the top layer of leaf tissue between the veins, giving the leaves a skeletonized appearance. Adults consume all leaf tissue between major veins, producing a distinctive lacy appearance to the foliage. The leaf veins remaining after Mexican bean beetle feeding often fall out due to wind or rain action, resulting in large, ragged holes in the foliage.
At mid pod fill, consider treatment when defoliation exceeds approximately 15 to 20% and the Mexican bean beetle is still present and actively feeding. More precise defoliation threshold guidelines are given in last week’s Pest&Crop to determine if treatment is justified.
![]()
Click for Table.
Black Light Catch Report
![]()
Downy Mildew on Soybean - (Andreas Westphal and Greg Shaner)
- This disease is more common this year.
Soybean leaves are receiving a lot of scrutiny this year. Many leaf diseases have flourished in the warm and humid conditions of the past few weeks. The careful observer will note several types of leaf spots, some of them more prevalent than others. Oval to circular yellow spots, about ¼ inch in diameter, have been seen in some fields (Fig. 1). These spots are the symptoms of downy mildew, caused by Peronospora manshurica. This fungus-like pathogen is omnipresent in soybean growing areas.

Figure 1. Downy mildew symptoms on the upper leaf surface.
The pathogen survives in soybean leaves and on soybean seed. In no-tillage systems and soybean monoculture, there is the higher potential for the pathogen to survive in the field. Infected soybean seed may exhibit a whitish coating on the seed coat. Primary infection occurs early in the season. When an infected seed germinates in cool and moist conditions, the pathogen infects the young seedling and invades the first trifoliolates. Once established in these, the pathogen produces sporulating structures on the underside of the leaf. Wind distributes these spores to other leaves. High humidity and moderate temperatures favor disease development. Younger soybean leaves are more susceptible than older leaves and will show the typical yellow halos that become larger and turn gray to finally be brown-necrotic (dead tissue). The pathogen grows inside the leaf tissue and sends spore-producing structures to the outside of the tissue. These sporulating structures can produce a grayish, fuzzy mass on the underside of the leaf just below the yellow spot visible on the upper leaf surface (Fig. 2). Although Peronospora manshurica is totally unrelated to the Asian soybean rust fungus, its spores are of similar size and shape to those of soybean rust. The spore-bearing structures of the downy mildew fungus have caused much confusion already because of the concern about soybean rust. Because of the threat of soybean rust and the economic consequences of applying fungicides, accurate diagnosis is more important today than ever.

Figure 2. Downy mildew symptoms on the lower leaf surface. Masses of sporangiophores can barely be distinguished at this magnification.
Downy mildew causes only limited damage to soybean crops and currently no treatment seems needed. The disease is considered to be the least damaging of all the soybean diseases known in the U.S. The recent high temperatures throughout Indiana are unfavorable for further spore production. As leaves become older, they are more resistant to infection.
The downy mildew pathogen is not a true fungus (it’s more closely related to the pathogens that cause potato late blight, tobacco blue mold, and downy mildew of grape). Foliar fungicides with full registration for use on soybean, for control of rust and other fungal diseases, will not control downy mildew (downy mildew is not mentioned on their labels). Fungicides that have Section 18 labels for use against soybean rust will likewise have little or no effect on downy mildew. In fields with high incidence of downy mildew this year, soybean should probably not be planted next year (there are many reasons, more important than downy mildew control, for not growing soybean after soybean). If for some reason soybean will be planted next year in a field where downy mildew was seen this year, tillage to hasten decomposition of soybean debris might be helpful.![]()
Frogeye Leaf Spot - (Gregory Shaner and Andreas Westphal)
- "Tropical" weather may bring this soybean disease farther north than usual.

Figure 1. Frogeye leaf spot lesions on soybean seen with incident light.
Frogeye leaf spot has been found in some Indiana soybean fields this summer. This disease, caused by the fungus Cercospora sojina, is more common in southern states, but in Indiana has seen extensively in the south in recent years. This year, frogeye leaf spot is at least as far north as central Indiana, but certainly not widespread. It is something to keep in mind when scouting fields for aphids and soybean rust.
Symptoms of frogeye leaf spot are fairly distinctive. Lesions are round to angular, up to 5 mm in diameter (Fig. 1). The lesion has a dark red brown border and a tan center. The surface of the lesion on the underside of the leaf is somewhat darker. Small, dark patches can be seen in the centers of lesions when they are examined with a hand lens. These are fascicles of dark conidiophores, the structures on which spores are produced. If an infected leaf is held up to the sky to provide backlighting, yellow halos can be seen around the dark lesions (Fig. 2). The dead tissue in the center of old lesions may fall out.
Leaves are most susceptible when they are young. Fully expanded leaves are much more resistant. All leaf layers may show frogeye, or there may be some layers with disease, and other layers with little or no disease—a reflection of different weather conditions as each successive leaf layer emerged. More growers are scouting fields this year than ever before, and because of the very humid weather of the past couple of weeks, there may be more sightings of frogeye leaf spot.

Figure 2. Frogeye lesions seen with blacklighting. Note yellow borders around the dark margins of the lesion.
There is resistance to frogeye leaf spot. Scott Abney, USDA-ARS soybean pathologist, is evaluating soybean varieties to each of several strains of the fungus. He has found that varieties that carry the Rcs3 gene have effective resistance against all of these strains.
In years past, frogeye leaf spot has not been serious enough to warrant control measures in most of Indiana. Several fungicides labeled for use on soybean, for example, chlorothalonil (Bravo, Echo), azoxystrobin (Quadris), and pyraclostrobin (Headline), will provide control of frogeye leaf spot. If a grower decides to use a fungicide to suppress the disease, fungicide labels will provide details about timing and rate of application.
![]()







Grain Fill Stages in Corn – (Bob Nielsen)?
The grain fill period begins with successful pollination and initiation of kernel development, and ends approximately 60 days later when the kernels are physiologically mature. During grain fill, the developing kernels will be the primary sink for concurrent photosynthate produced by the corn plant.
What this means is that the photosynthate demands of the developing kernels will take precedence over that of much of the rest of the plant. In essence, the plant will do all it can to “pump” dry matter into the kernels, sometimes at the expense of the health and maintenance of other plant parts including the roots and lower stalk. A stress-free grain fill period can maximize the yield potential of a crop, while severe stress during grain fill can cause kernel abortion and lightweight grain, and encourage the development of stalk rot.
Kernel development proceeds through several relatively distinct stages (Ritchie et. al., 1993).
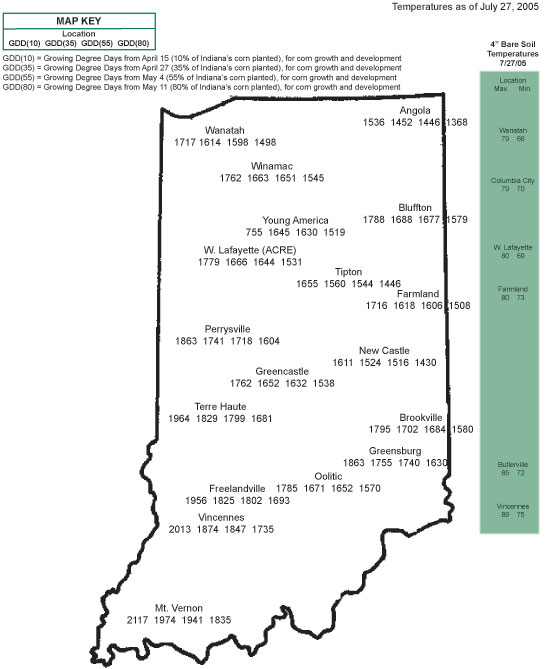
Silking Stage (Growth Stage R1)
Some may argue whether silking should be labeled as a kernel growth stage, but nonetheless silk emergence is technically the first identifiable stage of the reproductive period. Silks remain receptive to pollen grain germination up to 10 days after silk emergence (Nielsen, 2005a). Silk receptivity decreases rapidly after 10 days if pollination has not yet occurred. Natural senescence of silk tissue over time results in collapsed tissue that restricts continued growth of the pollen tube. Silk emergence usually occurs in close synchrony with pollen shed (Nielsen, 2005b), so that duration of silk receptivity is normally not a concern. Failure of silks to emerge in the first place (for example, in response to silkballing or severe drought stress) does not bode well for successful pollination.
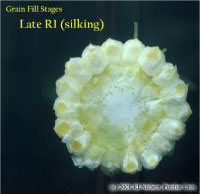
Kernel Blister Stage (Growth Stage R2)
About 10 to 14 days after silking, the developing kernels are whitish “blisters” on the cob and contain abundant clear fluid. The ear silks are mostly brown and drying rapidly. Some starch is beginning to accumulate in the endosperm. The radicle root, coleoptile, and first embryonic leaf have formed in the embryo by the blister stage. Severe stress can easily abort kernels at pre-blister and blister stages. Kernel moisture content is approximately 85 percent.
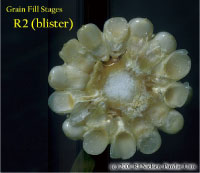
Kernel Milk Stage (R3)
About 18 to 22 days after silking, the kernels are mostly yellow and contain “milky” white fluid. The milk stage of development is the infamous “roasting ear” stage, that stage where you will find die-hard corn aficionados standing out in their field nibbling on these delectable morsels. Starch continues to accumulate in the endosperm. Endosperm cell division is nearly complete and continued growth is mostly due to cell expansion and starch accumulation. Severe stress can still abort kernels, although not as easily as at the blister stage. Kernel moisture content is approximately 80 percent.
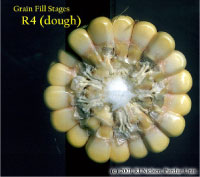
Kernel Dough Stage (R4)
About 24 to 28 days after silking, the kernel’s milky inner fluid is changing to a “doughy” consistency as starch accumulation continues in the endosperm. The shelled cob is now light red or pink. By dough stage, four embryonic leaves have formed and about 1/2 of the mature kernel dry weight is now in place. Kernel abortion is much less likely once kernels have reached early dough stage, but severe stress can continue to affect eventual yield by reducing kernel weight. Kernel moisture content is approximately 70 percent.
Kernel Dent Stage (R5)
About 35 to 42 days after silking, all or nearly all of the kernels are denting near their crowns. The fifth (and last) embryonic leaf and lateral seminal roots form just prior to the dent stage. A distinct horizontal line appears near the dent end of the kernel and slowly progresses to the tip end of the kernel over the next 3 weeks or so. This line is called the “milk line” and marks the boundary between the liquid (milky) and solid (starchy) areas of the maturing kernels. Severe stress can continue to limit kernel dry weight accumulation. Kernel moisture content at the beginning of the dent stage is approximately 55 percent.
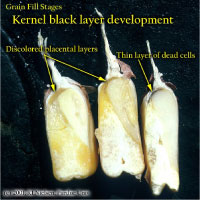
Physiological Maturity (R6)
About 55 to 65 days after silking, kernel dry weight usually reaches its maximum and kernels are said to be physiologically mature and safe from frost. Physiological maturity occurs shortly after the kernel milk line disappears and just before the kernel black layer forms at the tip of the kernels. Severe stress after physiological maturity has little effect on grain yield, unless the integrity of the stalk or ear is compromised (e.g., damage from European corn borer or stalk rots). Kernel moisture content at physiological maturity averages 30 percent, but can vary from 25 to 40 percent grain moisture.
Harvest Maturity
While not strictly a stage of grain development, harvest maturity is often defined as that grain moisture content where harvest can occur with minimal kernel damage and mechanical harvest loss. Harvest maturity is usually considered to be near 25 percent grain moisture.
Related References
Nielsen, R.L. (Bob). 2004. Yield Loss Potential During Grain Fill. Corny News Network, Purdue Univ. Online at www.kingcorn.org/news/articles.04/GrainFillStress-0705.html. (URL verified 7/5/04)
Nielsen, R.L. (Bob). 2005a. Silk Emergence. Corny News Network, Purdue Univ. Online at www.kingcorn.org/news/articles.05/Silks-0704.html. (URL verified 7/15/05)
Nielsen, R.L. (Bob). 2005b. Tassel Emergence & Pollen Shed. Corny News Network, Purdue Univ. Online at www.kingcorn.org/news/articles.05/Tassels-0704.html. (URL verified 7/15/05)
Ritchie, S.W., J.J. Hanway, and G.O. Benson. 1993. How a Corn Plant Develops. Iowa State Univ. Sp. Rpt. No. 48. Online at http://maize.agron.iastate.edu/corngrows.html. (last verified 7/5/04).
For other Corny News Network articles, browse through the CNN Archives at www.kingcorn.org/news/archive.html. For other information about corn, take a look at the Corn Growers’ Guidebook at www.kingcorn.org.
![]()