Pest & Crop Newsletter, Entomology Extension, Purdue University
Soybean Aphid Causing a Stir - (John Obermeyer, Christian Krupke, and Larry Bledsoe)
- Some early-planted soybean fields reported to have 20-30 aphids/plant.
- Insecticides applied with herbicides may be convenient but are NOT economic or efficacious.
- Drier conditions and lower predator numbers may have benefited soybean aphid.
- Insecticide treatment now ma lead to a second application later.
We have received several reports of V3-4 soybean plants with soybean aphid populations of 20 to 30 per plant. Most observations have come from northern counties, but a recent visit to southeast Indiana revealed their presence in an early-planted plot. Other states in the Midwest are reporting much the same thing. Because many producers are getting ready to apply post-emergence herbicides, they’re considering adding insecticide to the tank to save an application trip across the field. Please consider the following:
The economic threshold is 250 or more aphids per plant. Very few trials have been conducted in treating vegetative soybean for aphids at or below 250/plant, that data shows NO yield response. Aphid numbers of 20-30/plant is extremely low, and is not a certain indicator of economic populations later in the season. Furthermore, predators and parasites will begin to “take notice” of these numbers of aphids, and increase their feeding and foraging activities on these plants (more details below). Bear in mind that vegetative soybean are very resilient to damage of any kind, except in cases where plants are under extreme moisture stress.

Newly hatch ladybeetle larva feeding on soybean aphid.
Moisture-stressed soybeans throughout many areas of the state may have given aphid populations a boost this season. While winged aphids were first invading soybean this spring, many fields were lacking moisture. Aphids, like spider mites, thrive on the juices they obtain from drought stressed plants. This “high-protein” drink promotes healthy aphid growth and reproduction. Now that many areas have received much needed rainfall, it is possible that this population “surge” will subside.
Aphid predators may be down in numbers, but they are not out. There are many natural controls of aphids, and they are in every soybean field in the state. Though the numbers of predators are lower this spring they appear to be responding to increasing food availability…aphids! Soon predator numbers will increase and will have no problem controlling aphid numbers up to 100 individuals/plant. Obviously an insecticide sprayed this early in the season will eliminate most of these good guys. The predator’s ability to rebound is much slower than that of the soybean aphid after an insecticide application, and having to re-treat the field after an aphid resurgence is a real possibility.
Should dry conditions return, spider mite populations have potential to explode. Aphids and predators are not the only critters in the soybean fields this spring. Two-spotted spider mites have begun moving into thirsty fields. The recent rains did not kill them as is sometimes thought, but the rainfall does delay their growth and reproduction, in large part by promoting healthy bean plants. In a story similar to the one outlined above, spider mite populations can increase rapidly in the predator-free environment created following application of pyrethroid insecticides. Though the soybean aphid reproduction rate is impressive, it pales in comparison to spider mites!

Tiny syrphid larva sucking a winged aphid dry.
In summary, unless soybean fields are under extreme moisture stress, insecticides should not be applied for soybean aphid until the reproductive growth stages, and only after ≥250 aphids/plant has been reached. Treatments below these levels and early in the growing season will likely only serve to necessitate a need for second insecticide application later in the season. Happy scouting!
![]()
Rootworm Sampling in High Risk Fields - (John Obermeyer, Christian Krupke, and Larry Bledsoe)
- High-risk fields should be evaluated fr rootworm larva and damage.
- Insecticide efficacy on early-planted corn is suspect.
- Larval sampling procedures are outlined.
- Rescue treatment guidelines are given below.

Rootworm scarring and tunneling from early feeding.
Rootworm larvae have been hatching and seeking corn roots for over two weeks in northern counties and three or more weeks in southern counties. When first hatched the larvae are very small and live mostly within the roots. As they increase in size, so does their appetite. They will feed both inside and outside of the roots, causing tunneling and pruning. In our monitoring of soybean fields last season, we detected a large number of rootworm beetles especially in northern and central counties. It would be wise to sample roots of plants in these high-risk fields, especially where insecticide efficacy is in question (refer to Pest&Crop #9, 2005, “Early Planting and Rootworm Insecticide Efficacy”).
To sample for rootworms, use a shovel and lift out the root mass and surrounding soil and place on a dark surface (black plastic garbage bags work well). Carefully break up the clods and sort through the soil. Look for 1/4 to 1/2 inch long, slender, creamy-white larvae with brownish-black heads and tails. Once the soil has been separated from the root mass, inspect it for root scarring and pruning. You may find the larvae under the leaf collars that are close to nodal roots; tear these leaves away to check. Also, you may even observe the rootworms sticking out of roots. Repeat this process with several plants representing different areas of a field. An average of two or more larvae per plant represents a rootworm population that signals the need for a rescue cultivation application.
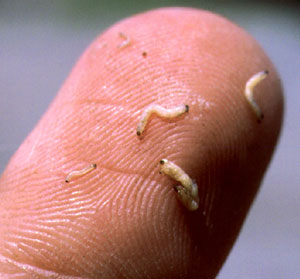
Different sizes of rootworm larvae.
Insecticides applied after planting should be directed toward the base of the plant. It is also important to throw soil up around plants to incorporate the insecticide and promote the establishment of brace roots. A good brace root system will help prevent plant lodging and reduce yield losses due to rootworm feeding. If a no-till field has an economic population of larvae, placing the insecticide on top of the ground will usually not be effective. The only exception may be if the soil insecticide is watered in through irrigation or rainfall (ideally ½” or more). Two liquid soil insecticides, Furadan 4F and Lorsban 4E, are labeled for post-emergent applications. If one decides to mix the insecticide with a liquid nitrogen source for a side-dress application, compatibility checks should be made. Broadcasting the insecticide will greatly diminish rootworm efficacy.
A QuickTime movie of rootworm larval sampling can be viewed at www.entm.purdue.edu/entomology/ext/fieldcropsipm/videos.htm.
![]()
Click for Table.
Black Light Catch Report
![]()
Musk Thistle and Its Control – (Glenn Nice and Bill Johnson )
- Cultural Control
- Chemical Control
- Biological Control
When in full bloom, musk thistle, also known as nodding thistle, is an impressive plant. That does not mean we want it in our pastures or in our yards. Its tall stem with a large purple flower appear in many of our pastures, ditches, and waist areas. The stem will often droop from the weight of the flower, thus the name nodding thistle. We are not alone in having to deal with this established visitor of Eurasia, for Australia and Argentina also have problems with musk thistle in their pastures. Musk thistle is on several states noxious weed lists.

Figure 1. Musk thistle developing flower. Note the spines on the wings on the stem.
Cultural Control
A pasture that is well maintained and competitive can suppress musk thistle establishment. Often pastures that have been over grazed or not well maintained will have problems with musk thistle. We often see musk thistle in waste areas that have not been mowed regularly. A long term strategy is to keep it from going to seed. Mowing pastures during stem elongation, but before flowering can inhibit seed production. In many cases expect regrowth; so a second mowing event will most likely be necessary. To inhibit infestation again, also prevent musk thistle from growing along fence rows and waste areas and going to seed. If you find yourself clipping the flowers, dispose of them in plastic bags.
Chemical Control
Unfortunately the best time to control musk thistle is when we don’t notice it. Optimum time of control is in the fall or early spring when it is in the rosette stage, before it bolts. Do not apply when rosettes are under stress by drought or excessive temperatures. Many of the products that have activity on thistles require the plant to be actively moving sugars from the leaves into the root stocks. Not controlling the root stocks will result in regrowth. There are several herbicides that are active on musk thistle. For a list of herbicides see Table 1. Many of the products that are excellent on musk thistle are from the growth regular group of herbicides. These are generally labeled for established grass pastures and will injure any clover that might be mixed with the grasses. In a legume grass pasture you are restricted to the use of glyphosate as a spot or rope wick application.
Biological Control
Musk thistle is a good candidate for biological control. It is a weed that is aggressive and on the increase, but generally affects marginal low economic valued lands. Two weevils have been used in the past. The seed head weevil (Rhinocyllus conicus) lays its eggs in the flowers. The eggs hatch and the larvae feed on musk thistle’s seed. Another weevil used for biocontrol is the rosette crown weevil (Trichosirocalus horridus). This weevil feeds on the crowns of musk thistle. Fungi have also been released for the control of musk thistle. The rust, Puccinia carduorum was released in western Virginia in 1987. For a list of insect and fungi that have been investigated as biocontrol agents for musk thistle see the following web site on The Invasive Species web site www.invasive.org/eastern/biocontrol/18MuskThistle.html. In most cases an integrated management approach is required
Reference:
Richard W. Medd and Richard C. G. Smith. 1978. Prediction of the potential distribution of Carduus nutans (nodding thistle) in Australia. Journal of Applied Ecology 15:603-612.
James Stubbendieck, Mitchell J. Coffin, and L. M. Landholt. 2003. Weeds of the Great Plains. Nebraska Department of Agriculture. pp 96 and 97.
Merrill A. Ross and Daniel J. Childs. 1993. Musk Thistle Control in Permanent Grass Pastures. Purdue University Cooperative Extension Service. WS-19 (out of print).
M. M. Loux, J. M. Stachler, W. G. Johnson, G. R. W. Nice, and T. T. Bauman. 2005. Weed Control Guide for Ohio and Indiana.
Musk Thistle Biological Control Plan. Accessed June 9th, 2005. The Colorado Department of Agriculture. www.ag.state.co.us/DPI/publications/muskthistle.html.
A. B. A. M. Baudoin and W. L. Bruckart. 1996. Population dynamics and spread of Puccinia carduorum in the eastern United States. Plant Disease. 80:1193-1196.
![]()
Leaf Blotch and Leaf Rust of Wheat - (Gregory Shaner)
- A late season flush of disease may have some effect on yield and grain quality.
On June 8, I assessed disease severity in wheat plots at the Southeast Purdue Ag Center in Jennings County. Disease severity was moderate. There was some stripe rust on flag leaves of some cultivars. All cultivars had symptoms of Stagonospora leaf blotch on lower leaves, including the leaf below the flag, but little or no leaf blotch on the flag leaf. I was back at SEPAC on June 13, and found Stagonospora blotch to be severe on flag leaves. On many cultivars, 40% or more of the flag leaf area was necrotic. Most of the lesions were still small, because they probably had appeared only during the previous couple of days. The lesions were elliptical, with tan centers and yellow borders—the classical Stagonospora leaf blotch symptom.
Rain fell on 12 of the past 30 days, with rain days dispersed throughout this period. The infections now evident on flag leaves may have occurred during rains that fell from May 27 through June 3.
Loss of flag leaf tissue as the result of infection this late in the season will not be as damaging as it would had infection occurred earlier, but given that the grain is still in the late milk stage of development there will probably be some reduction in test weight and yield.
In central and northern Indiana, leaf rust has become severe on some cultivars. Pustules cover much of the flag leaf on susceptible cultivars. Here too, some reduction in yield and test weight will result from disease. Many cultivars available for production in Indiana have good resistance to leaf rust. Every year there is a threat of leaf rust, so it’s best to avoid cultivars that are susceptible.
![]()
Soybean Rust - (Gregory Shaner)
- Did Arlene bring something besides rain to Indiana?
Tropical storm Arlene moved from the Gulf, into the southern U.S. and then on up into Kentucky, Illinois, and Indiana. The track of this storm would seem to be ideal for carrying spores of the Asian soybean rust fungus into our area. But, to carry spores of Phakopsora pachyrhizi north, there would need to have been an abundant source of spores somewhere along Arlene’s track. We are fairly confident that there were no areas of intense rust development in the continental U.S. During the winter and spring, plant pathologists throughout the South have been monitoring soybean fields, sentinel plots, and kudzu sites. To date, Asian soybean rust has been found only in four counties in Florida, where the fungus overwintered on kudzu, and two counties in southwest Georgia, on volunteer soybean. These areas are to the east of the path of Arlene as the storm made its way from the Gulf through Mississippi, Alabama, Tennessee, Kentucky, and Indiana. Scouting in Mississippi and Alabama this spring has not revealed any rust. Although the storm track posed a potential threat for Indiana, the absence of rust in these southern states suggests that there were very few spores that could have been picked up by these southerly winds and transported into Indiana.

Figure 1. The leaf shows early symptoms of Asian soybean rust. The infection sites are small, dark spots with no yellow border. The larger dark spots with a distinct yellow halo are probably bacterial blight.
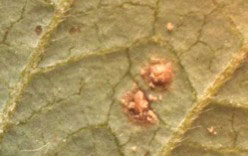
Figure 2. Close-up view of a soybean rust pustule. The pustule is erumpent and is covered with spores of the fungus.

Figure 3. Early brown spot lesions. Note that some of these spots have a yellow border, but others do not. The ones without a yellow border would be very difficult to sitinguish from soybean rust at this stage.

Figure 4. When brown spot lesions mature and become more numerous, the leaf becomes generally yellow and necrotic areas become larger, as lesions coalesce. At this stage, brown spot can be readily distiguished from rust.
An uncertainty about the transport of soybean rust spores by Arlene is the amount of inoculum in Central America and the Caribbean. There have been no reports of severe outbreaks of rust in these areas. However, the fungus has a broad host range that includes many tropical and subtropical legumes that are not crop plants. Spores from these hosts could have been carried into the U.S., but their numbers are likely to be low.
Although Arlene probably did not carry a large number of rust spores into Indiana, it is possible that the storm carried some. Therefore, it would be prudent to start scouting soybean fields in Indiana. If low numbers of spores were carried into Indiana during the weekend of June 11, we would not expect to see the pustules that develop from this inoculum for about 9 days. That’s how long it takes for a spore to infect and produce a new pustule. Early next week, about June 20, would be the time to start scouting.
If any infections result from spores carried into Indiana by Arlene, they will likely be few. To detect initial infections, at least 150 leaves in a field should be examined carefully for rust. Pustules develop primarily on the underside of the leaf, so this is where to look. Examine leaves in the middle and lower canopy. Upper leaves will have expanded after any infection that might have occurred this past weekend, and would not have been exposed to spores. Examine leaves three or more nodes below the uppermost node. To scout a field, stop at 10 locations, following an irregular path, and examine 15 middle and lower leaves. Any leaf with a suspicious spot should be detached and examined with backlighting, by holding the leaf up against the sky. Rust infections are initially evident as small, dark spots against a green background (See Fig. 1). It may help to circle any suspicious spots with a marker pen. Then, use oblique, incident light to look at the spot with a hand lens. A good quality lens of 10x magnification is sufficient, but a 15x or 20x lens is better. If rust pustules are present, these can be discerned with a hand lens. The pustule is erumpent. Although individual spores cannot be distinguished with a hand lens, en masse they are evident as a small, granular mass of material. When spores are not abundant on the pustule, the pustule itself is visible as a conical protrusion with a small hole at the apex. Before the rust infection has progressed to the development of a mature pustule, it is much more difficult to make a diagnosis. Initially, rust infections appear as small dark spots (Fig. 2). Spots such as these could result from several causes. Leaves with suspicious spots can be put in a plastic bag with a damp paper towel. Inflate the bag by blowing into it, and then tie the top. Allow the leaves to incubate for a couple of days at room temperature, out if direct sunlight. If the lesions are the result of rust, pustules will develop.
The major rust look-alike disease that will likely be seen on lower leaves of soybean is brown spot. When lesions of this disease are fully developed, they look quite different from soybean rust pustules (Fig. 3). However, when brown spot lesions are first developing, they are small, dark purple lesions that could be mistaken for a rust infection that has not yet matured into a pustule (Fig. 4). Because of the dry and cool weather during late May and early June, there is less brown spot on soybean leaves than usual. Soybeans that were planted early show brown spot on the unifoliolate leaves and lower trifoliolates, but later plantings are not yet showing brown spot symptoms.
If symptoms are seen that suggest rust infection, leaves (about 20, if possible) should be sent to the Plant and Pest Diagnostic Laboratory at Purdue. Special soybean rust submission forms can be obtained from County Extension Offices, or can be downloaded from the Rust ID/sample submission “button” on the PPDL web site www.ppdl.purdue.edu/ppdl/soybean_rust.html. Place the leaves between sheets of newspaper and put them in a sealable plastic bag. Do not add water! Write the submitter’s name and field identification on the plastic bag. Put this bag in a second sealable plastic bag, and enclose this and the sample submission form in a box or envelope and hand deliver or ship overnight to the Purdue Plant and Pest Diagnostic Laboratory. The shipping address can be found on the sample submission form.
![]()