Pest & Crop Newsletter, Entomology Extension, Purdue University
Winter Temperatures, Corn Flea Beetle Survival, and Potential for Stewart’s Wilt - (Christian Krupke, John Obermeyer and Kiersten Wise)
- Corn flea beetle winter survival is expected to be low in northern and central Indiana.
- Moderate survival is expected for southern Indiana, higher in the Ohio River valley.
- Corn flea beetle is a vector of Stewart’s wilt of corn.
- Management guidelines are given below.
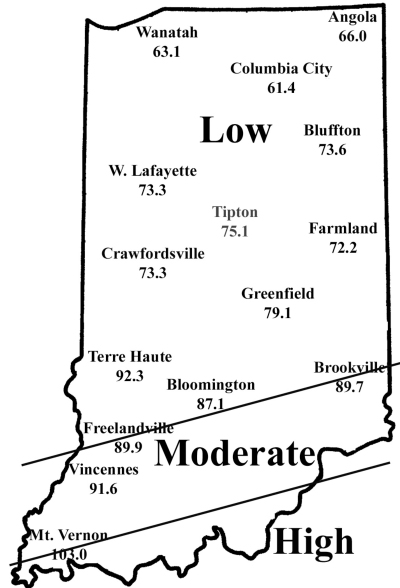
Corn flea beetle is a sporadic corn pest in Indiana. Winter temperatures in regions where beetles were abundant last season will determine if there is cause to be concerned this season. This is especially important since this insect transmits the bacterium that causes Stewart’s disease in corn. The severity of the disease correlates well with winter temperatures because the organism survives in the gut of the overwintering beetles. Warmer temperatures result in higher beetle survival, and therefore a greater potential for Stewart’s disease. To determine the potential severity of Stewart’s disease, add the average daily temperatures for the months of December, January, and February. If the sum is below 90, the potential for disease problems to develop is low. If between 90 and 100, moderate disease activity is a possibility. Sums above 100 indicate a high probability that beetles will survive the winter and vectoring of Stewart’s disease will occur. To help you better gauge the potential for corn flea beetle activity in your area (and the potential severity of the threat of the disease) in 2009, we have created the state map shown below. According to the temperature model, there is low probability of corn flea beetle activity and subsequent disease in northern and central Indiana, and moderate activity in areas south of US 50.
This temperature model for corn flea beetle has been in use for many years and has been fairly accurate in predicting the activity of this pest the following spring. However one inherent flaw is that the model is based on ambient air temperatures, not temperatures under leaf litter and grass clumps where this pest is actually overwintering. If snow cover is present, this provides an insulating blanket for the insect, and may protect some beetles from winterkill. Even with this “disclaimer” statement, we think the 2008/2009 winter was cold enough to have negatively impacted overwintering beetles in most of Indiana. Also, flea beetle numbers have been low statewide, in general, for the last 3-4 years.
As for the disease, there are two phases of Stewart’s wilt: a seedling wilt phase and a leaf blight phase. In the wilt phase, plants wilt rapidly, usually at an early stage of growth. Leaves emerging from the whorl of infected plants are often the first to wilt. Internal tissues at the growing point are discolored or hollowed out. Faint green to yellow streaks containing corn flea beetle feeding marks are visible on one or more leaves. If stalks of wilted plants are cut, it may be possible to see yellow beads of bacteria ooze from the vascular tissue. Sweet corn hybrids are especially susceptible. Some dent corn inbreds, and occasionally dent corn hybrids, and some popcorn lines are susceptible as well. Dent corn hybrids rarely show symptoms of the wilt phase after growth stage V5.
The leaf blight phase can occur at any time during the growing season, but often does not appear until after tasseling. Lesions are typically long and narrow, with greenish-yellow streaks and irregular or wavy-margins. Lesions will become straw-colored, and infected leaves may die prematurely. Hybrids with resistance to Stewart’s wilt may have smaller lesions that are limited to the tissue surrounding the feeding site of the beetle. These lesions can be confused with the fungal diseases gray leaf spot or northern corn leaf blight. Stewart’s wilt is also commonly confused with another bacterial disease, Goss’s wilt. One way to differentiate between these diseases and Stewart’s wilt is to look for the beetle feeding scars associated with Stewart’s wilt.
Management decisions made now should be based on the corn’s susceptibility to the disease and anticipated risk.
Low susceptibility/risk - pest managers should scout fields and apply a foliar insecticide after emergence if (1) beetle feeding damage becomes severe, (2) there are 5 or more beetles per plant, and (3) seedlings are growing slowly (e.g., cool temperatures).
High susceptibility/risk - sample field edges and in-field areas of grass weed residue (i.e., overwintering sites) before planting to assess overwintering beetle survival and potential beetle movement to emerging corn plants. A sweep net is an ideal sampling tool for this pest. If any beetles are discovered at this time, an at-planting insecticide application is warranted. Most of the corn seed currently sold in Indiana is already protected from corn flea beetle at the time of purchase: Cruiser and Poncho insecticide-treated seed are systemic insecticides that should give good control of flea beetle in the early seedling stage. The low rates of the seed treatments are expected to provide protection from emergence to 2-leaf corn, whereas the higher rate (eg. Poncho 1250 and Cruiser 1.25, also called the “rootworm rate”) should protect corn through the 5th leaf stage.
If insecticide-treated seed is not an option, broadcast application of foliar insecticides at the time when corn spikes should provide 7-10 days of residual protection from beetle feeding.
CAUTION: treating of field edges and waterways for beetle control may be an off label application. Avoid movement of insecticides, including soil-bound materials into aquatic environments.
Expected Flea Beetle Winter Survival
| Disease Site | Dec. | Jan. | Feb. | Sum | Threat |
| Angola | 24.4 | 14.6 | 27.0 | 66.0 | Low |
| Wanatah | 21.5 | 15.0 | 26.6 | 63.1 | Low |
| Bluffton | 26.2 | 19.2 | 28.2 | 73.6 | Low |
| W. Lafayette | 25.5 | 17.9 | 29.9 | 73.3 | Low |
| Tipton | 27.0 | 19.0 | 29.1 | 75.1 | Low |
| Farmland | 26.1 | 18.1 | 28.0 | 72.2 | Low |
| Crawfordsville | 27.7 | 16.1 | 29.1 | 73.3 | Low |
| Greenfield | 27.8 | 21.4 | 29.9 | 79.1 | Low |
| Terre Haute | 29.9 | 26.5 | 35.9 | 92.3 | Moderate |
| Brookville | 31.1 | 25.6 | 33.0 | 89.7 | Moderate |
| Bloomington | 30.2 | 23.5 | 33.4 | 87.1 | Low |
| Freelandville | 31.5 | 24.5 | 33.9 | 89.9 | Moderate |
| Vincennes | 32.3 | 23.8 | 35.5 | 91.6 | Moderate |
| Mt. Vernon | 35.6 | 30.4 | 37.0 | 103.0 | High |
Identification of Six Alternative Winter Annual Weed Hosts and One Cool Season Perennial Host for Soybean Cyst Nematode – (Valerie Mock, Bill Johnson)
There are six winter annual weeds and one cool season perennial that have been identified as alternate hosts to soybean cyst nematode. We have observed that SCN can reproduce in the field on purple deadnettle. Fields with these weed hosts may be increasing SCN population densities at a faster rate than fields without weed hosts. A recent study in Indiana found that known SCN weed hosts were prevalent in 93 percent of the fields surveyed (Creech and Johnson, 2006), indicating the possibility of a statewide increase in nematode population densities due to weeds. In Indiana SCN has been found in 82 of 92 counties (Faghihi, et. al., 2006).
Most of these weeds can start to emerge during late august and September. So consider using this guide to scout fields and determine if you have these weeds present and if the density or future cropping plans would warrant fall treatments for winter annual weeds.
The purpose of this article is to identify characteristics of each of the six winter weed hosts and the one cool season perennial. We will discuss them in the order of strongest to weakest host.
Winter annual weed hosts:
•Purple deadnettle (strong host)
•Henbit (strong host)
•Field pennycress (moderate host)
•Shepherd’s-purse (weak host)
•Small-flowered bittercress (weak host)
•Common chickweed (weak host)
Cool season perennial:
•Mouseear chickweed (weak host)
Purple deadnettle and henbit are strong hosts and it can be difficult to distinguish the two weeds when they are small.
Purple Deadnettle (Lamium purpureum)
Leaves: Cotyledons have a white tip, and are oval with a notch where the petiole connects to the cotyledon. Leaves have prominent venation, resulting in a crinkled look. Leaves at the base of the stem are hairy and circular in shape. Leaves at the top of the stem are hairy and triangular in shape.
Stems: Square and greenish-purple in color. Stems tend to branch at the base of the plant and have hairs that point downward.
Flowers: Blooms are purple, and occur in upper leaves in whorls of 3 to 6. Purple deadnettle blooms between April and October.
Purple deadnettle (young)
Henbit (Lamium amplexicaule)
Leaves: Cotyledons are oval, have a white tip, and notched where the cotyledon and petiole meet. Leaves are circular with rounded teeth along edges and slight venation which causes a crinkled look. Leaves have hair on their upper surfaces and hair along the veins on their lower surfaces. Leaves at the top of the stem wrap around the stem and are sessile.
Stems: Stems are square, tend to branch near the base of the plant, have hairs that point downward, and are green or purple.
Flowers: Flowers are purple to pink. Henbit can flower from March to November.
Henbit (young)
Field pennycress and shepherdspurse are weaker hosts than purple deadnettle and henbit. Much like purple deadnettle and henbit, these weeds can be difficult to distinguish from one another.
Field Pennycress (Thlaspi arvense)
Leaves: Cotyledons are oval and have a bluish-green tinge with a long petiole. When the basal rosette forms, the leaf margin is wavy and slightly toothed. Leaves are egg-shaped, light green, and hairless. At maturity, no basal leaves are present. After bolting, leaves on the stem are oblong to lanceolate, have no petioles, have toothed leaf edges, and lobes that are pointed and clasp stem. Leaves emit a strong odor when disturbed.
Stems: Stems have no hairs. The leaves generally fall off the stem as the plant matures and can be branched in the top part of the stem.
Flowers: Flowers are white with four petals. Field pennycress flowers from April through June, and has a round seed pod (silicle) with a winged margin that is notched at the tip.
Field Pennycress (rosette)
Field Pennycress (pods)
Shepherd’s-purse (Capsella bursa-pastoris)
Leaves: Cotyledons have long petioles, and are egg shaped to round and narrower at the base. Young leaves are round and slightly hairy on surface with slightly toothed margins.
Generally, deeply lobed to deeply toothed leaves form around the 5th to 7th leaf and are dark green to silvery-gray.
Stems: Stems are unbranched and slender with gray hairs.
Flowers: White with four petals. Flowers from spring to early summer and sometimes in autumn. Seed pod is heart-shaped.
Shepherd's-purse (rosette)
Small-flowered bittercress (Cardamine parviflora)
Leaves: Basal leaves are deeply lobed, which gives the appearance of 3-6 pairs of leaflets with a rounded terminal leaflet.There are 4-10 stem leaves and no basal leaves at maturity. Leaves are generally hairless, but can be slightly hairy.
Stems: Branched with some leaves.
Flowers: Flowers are white with four petals. The seed capsule is a silique, which is long and narrow.
Small-flowered bittercress (rosette)
Common Chickweed (Stellaria media)
Leaves: Cotyledons are slender and ovate. Young leaves are opposite and round to egg-shaped with pointed tips. Mature leaves are oppositely arranged, light green, egg-shaped, pointed at the tips, and hairless. Hairy petioles occur on most leaves, but petioles are not present on the upper leaves.
Stems: Stems start to branch when five leaf pairs form. Stems are light green and generally smooth, but may have 1-2 rows of hairs.
Flowers: Flowers have five white petals that bloom from early spring to autumn.
Common Chickweed (seedling)
Mouseear Chickweed (Cerastium vulgatum)
Leaves: Cotyledons are round or ovate and generally lack hairs. Hairs may be found on the base of the stem. Young and mature leaves are opposite, dark green, and hairy.
Stems: Stems have two rows of hairs and when nodes contact the soil they form roots.
Flowers: Flowers have five white petals that bloom from May through October.
It is important to reemphasize that these weeds can be found in a large percentage of no-till or reduced till fields in Indiana, and that SCN has been found in 82 of 92 counties in Indiana. We have conducted a number of field, greenhouse, and lab studies, funded by the Indiana Soybean Alliance and the USDA, to investigate the interaction between these winter weeds and SCN. If you have any of these 6 hosts, and particularly the strong hosts, you should be concerned about management of these weeds and how that may impact soybean profitability.
Glossary
basal rosette: Leaves radiating from the stem of the plant in a circular cluster at ground level.
cotyledon: The seed leaf.
leaflet: One subunit of a compound leaf.
petiole: The stalk between the stem and leaf blade.
sessile: Lacking a petiole.
silicle: Fruit of the Brassicaceae that is not much longer than wide (if at all).
silique: Fruit of the Brassicaceae that is an elongated capsule.
terminal leaflet: Occurs at the tip of the main compound leaf as a single subunit.
winter annual: Plant that germinates in late summer to early spring, flowers, produces seed in mid- to late spring, then dies.
References
Creech, J. E., and W. G. Johnson. 2006. Survey of broadleaf winter weeds in Indiana production fields infested with soybean cyst nematode (Heterodera glycines). Weed Technol. 20:1066-1075.
Bradley, K., and S. Hagood. Virginia Tech Weed Identification Guide. <http://ipm.ppws.vt.edu/weedindex.htm>. Accessed: 6/22/2007.
Faghihi, J., and V. R. Ferris. 2006. Soybean cyst nematode. Department of Entomology. Purdue University. Web page: <http://extension.entm.purdue.edu/publications/E-210.pdf> Accessed: 6/22/2007.
Uva, R.H., J.C. Neal, and J.M. DiTomaso. 1997. Weeds of the Northeast. Cornell University Press. South Korea. pp. 172-248.
Photo Source Kevin Bradley, Earl Creech, and Valerie Mock
![]()
Spray Drift Caution - (Tom Jordan, Bill Johnson, Tom Bauman, and Glenn Nice)
As the new cropping season approaches, everyone should be aware of the impact that herbicide drift can cause to off-target crops. Indiana is a highly populated state, and one of the fastest growing sectors is small specialty crop farms, including vegetables, grapes, and greenhouses and high tunnels. High tunnels are usually plastic greenhouses without built-in heaters or fans. These structures allow producers to start growing plants in late February or early March, and many will be in operation by the time burndown and early herbicide treatments are being made to agronomic crops.
While these specialty crop producers should know that corn and soybean fields will be sprayed at this time of year and should take precaution to protect their crops from drift, it is also the responsibility of those who treat agronomic crops to not let their applications drift off target.
A check with the State Chemist’s Office showed that complaints from specialty crop growers are on the rise. Of all the spray drift complaints that they received last year, 79% were from applications made to agronomic crops. Of all the agronomic crop drift complaints, 67% were from commercial applications and 25% from private applications. The rest came from a variety of other types of applications including rights of ways, roadsides, and others. These percentages are reasonable, given the way herbicide applications are made in Indiana.
High value specialty crops like vegetables, grapes, and flowers are damaged more severely from spray drift than are corn and soybeans, and have less chance to recover. This is especially true for growth regulator herbicides. Usually the crop is not suitable for marketing. With 6.4 million people in this small state, the specialty crops sector of agriculture will continue to grow. It is imperative that wind speeds and directions be considered when applying herbicides near vegetable fields and grape vineyards. If greenhouses or high tunnels are visible near enough to fields that drift onto or into them is a potential, maybe a good neighbor practice is to tell the owner you are about to spray in order to give them time to turn off fans and/or close the sides of their structures.
Drift will never be eliminated and spray conditions will never be perfect, but doing all that is possible to keep spray drift off high value crops will go a long way toward cutting down on complaints and lawsuits.
Field Crop Diseases Recap for 2008 and Forecast for 2009 – (Kiersten Wise)
Despite favorable weather conditions for disease development in June, 2008 was a relatively quiet year for diseases of corn and soybean in Indiana. Common rust was frequently observed in fields throughout the state, but typically at low levels. Gray leaf spot was present at low levels in most fields late in the season, but moderate to severe infection was generally only observed on susceptible hybrids. Late season stalk rots and Diplodia ear rot were more prevalent in 2008 than in past years.
We did find Goss’s bacterial wilt of corn for the first time in Indiana in 2008. The disease was identified on popcorn and hybrid corn in Jasper and Pulaski counties in northwest Indiana, and led to considerable yield loss in affected fields. Goss’s wilt is sporadic throughout the Midwest, but usually appears only in limited areas, and is manageable through crop rotation and tillage.
Soybean diseases in Indiana were less severe than in recent years, although some diseases were locally severe. Sudden death syndrome (SDS) was widespread throughout Indiana this year due to cool, wet soil conditions after planting. Wet weather at flowering contributed to white mold outbreaks in northeastern and central Indiana, and substantial yield loss was observed in some fields. Dry conditions late in the growing season in northeastern Indiana may have contributed to the development of charcoal rot. Charcoal rot symptoms were widespread and severe in this region, and reduced soybean yields. Fungal structures produced by the white mold and charcoal rot fungi can survive in the soil for several years, and crop rotation can help reduce the future risk of these diseases in affected fields.
Soybean rust was not detected in Indiana in 2008. The disease developed slowly in the southern U.S. in 2008, and weather patterns were not conducive for soybean rust spore movement into Indiana during the critical soybean growth stages (R1 to R6). However, if rust were to develop and spread in the southern states at an earlier time next year, spores could reach the Indiana soybean crop at a stage where yield loss could occur.
The most important and unpredictable variable for determining risk of soybean rust infection, and all of our field crop diseases in Indiana, is the weather. It is hard to determine which diseases will require our attention in 2009, but rest assured we will continue to monitor fields for disease problems and let you know what is out there!
![]()
Cover Crop Advantages, Acceptance and Challenges – (Barry Fisher and Tony Bailey, USDA NRCS with Christian Krupke and Bill Johnson)
- Cover crops provide agronomic benefits and economic incentives.
- Cover crop acreage has recently increased dramatically.
- Insect and weed management issues are addressed below.
Cover crops have been used for many different purposes over the years including erosion control, nitrogen fixation and weed suppression. More recently, cover crops have also been promoted for increasing and encouraging no-till adoption, improving soil health, increasing soil organic carbon, retaining nitrogen over the fall and winter, supplemental forage production and after manure applications.
USDA’s Natural Resources Conservation Service (NRCS), through the Environmental Quality Incentives Program (EQIP), has offered payments to eligible and interested producers to plant cover crops. In 2008, EQIP paid producers $ 22.50/acre to plant cover crops and possibly more if tied to the Energy Conservation System or manure waste utilization.
The amount of cover crops planted has increased drastically. In 2005 13,000 acres were EQIP contracted, 15,000 acres in 2006, 76,000 acres in 2007 and (drum roll please) 160,000 acres in 2008. The most popular types of cover crops are annual ryegrass, oilseed radish, oats, cereal rye, vetches and clovers.
Cover crops are not without their challenges. Those that stay green late into the spring, can also attract insects and other pests that may need to be controlled, and will require additional crop scouting. The most commonly found insects include the migratory pests black cutworm and armyworm (particularly common on annual ryegrass) – the females of both species are capable of migrating long distances and will deposit eggs in a wide range of hosts. These caterpillars often reach high densities in the cover crop and will often move from the cover crop to the row crop if there is not a sufficient “host free period.” To decrease the chances of insect infestation, the cover crop should be killed at least 2 weeks prior to planting the row crop. Regrowth of the cover crop should be treated with an appropriate herbicide mixed with a pyrethroid insecticide. Because reinfestation and hatching of new eggs may occur after treatment, additional scouting for insects will still be needed at, and shortly after, plant emergence to determine whether further insecticide treatments are required.
An additional challenge is the termination of the cover crop at the time needed. While several crop selections will winter kill, such as oats and oilseed radish, many will live through the winter and need a herbicide application for control sometime in the spring prior to planting row crops. For more information on how to control cover crops consult Table 1 on page 16 of the 2009 Ohio and Indiana Weed Control Guide (WS 16) <http://www.btny.purdue.edu/Pubs/WS/WS-16/BurndownTable.pdf>.
PURDUE EXTENSION FIELD CROP SPECIALISTS
| Entomology: http://extension.entm.purdue.edu/ | |||
| Steve Yaninek | (765) 494-4554 | yaninek@purdue.edu | Head, Dept. of Entomology |
| Larry Bledsoe | (765) 494-8324 | lbledsoe@purdue.edu | Field Crop Insects, CAPS |
| Jamal Faghihi | (765) 494-5901 | jamal@purdue.edu | Nematology |
| Greg Hunt | (765) 494-4605 | hunt@purdue.edu | Beekeeping |
| Christian Krupke | (765) 494-4912 | ckrupke@purdue.edu | Field Crop Insects |
| Judy Loven | (765) 494-8721 | loven@purdue.edu | USDA, APHIS, Animal Damage |
| Linda J. Mason | (765) 494-4568 | lmason@purdue.edu | Food Pest Mgmt. & Stored Grain |
| John L. Obermeyer | (765) 494-4563 | obe@purdue.edu | Field Crop Insects & IPM Specialist |
| Tammy Luck | (765) 494-8761 |
luck@purdue.edu | Administrative Assistant |
| Agronomy: http://www.agry.purdue.edu/ext | |||
| Craig Beyrouty | (765) 494-4774 | beyrouty@purdue.edu | Head, Dept. of Agronomy |
| Sylvie Brouder | (765) 496-1489 | sbrouder@purdue.edu | Plant Nutrition, Soil Fertility, Water Quality |
| Jim Camberato | (765) 496-9338 | jcambera@purdue.edu | Soil Fertility |
| Corey Gerber | (765) 496-3755 | gerberc@purdue.edu | Director, Diagnostic Training Center |
| Brad Joern | (765) 494-9767 | bjoern@purdue.edu | Soil Fertility, Waste Mgmt. |
| Keith D. Johnson | (765) 494-4800 | johnsonk@purdue.edu | Forages |
| Charles Mansfield | (812) 888-4311 | cmansfie@purdue.edu | Small Grains, Soybean, Corn |
| Robert L. Nielsen | (765) 494-4802 | rnielsen@purdue.edu | Corn, Sorghum, Precision Agriculture |
| Gary Steinhardt | (765) 494-8063 | gsteinha@purdue.edu | Soil Mgmt., Tillage, Land Use |
| Tony Vyn | (765) 496-3757 | tvyn@purdue.edu | Soil Mgmt. & Tillage |
| Terry West | (765) 494-4799 | twest@purdue.edu | Soil Mgmt. & Tillage |
| Lisa Green | (765) 494-4783 |
lmetts1@purdue.edu | Extension Secretary |
| Botany and Plant Pathology: http://www.btny.purdue.edu/Extension | |||
| Peter Goldsbrough | (765) 494-4615 | goldsbrough@purdue.edu | Head, Dept. of Botany & Plant Pathology |
| Tom Bauman | (765) 494-4625 | tbauman@purdue.edu | Weed Science |
| Tom Creswell | (765) 494-7071 | cresswell@purdue.edu | Director, Plant & Pest Diagnostic Lab |
| Dan Egel | (812) 886-0198 | egel@purdue.edu | Southwest Purdue Ag Center |
| William Johnson | (765) 494-4656 | wgj@purdue.edu | Weed Science |
| Tom Jordan | (765) 496-2078 | tjordan@purdue.edu | Weed Science |
| Glenn Nice | (765) 496-2121 | gnice@purdue.edu | Weed Science |
| Gail Ruhl | (765) 494-4641 | ruhlg@purdue.edu | Plant & Pest Diagnostic Lab |
| Greg Shaner | (765) 494-4651 | shanerg@purdue.edu | Diseases of Field Crops |
| Fred Whitford | (765) 494-4566 | fwhitford@purdue.edu | Purdue Pesticide Programs |
| Kiersten Wise | (765) 496-2170 | kawise@purdue.edu | Diseases of Field Crops |
| Charles Woloshuk | (765) 494-3450 | woloshuk@purdue.edu | Mycotoxins in Corn |
| Amy Deitrich | (765) 494-9871 FAX: (765)494-0363 |
amymd@purdue.edu | Extension Secretary |
| Agricultural & Biological Engr.: http://pasture.ecn.purdue.edu/ABE/Extension/ | |||
| Bernie Engel | (765) 494-1162 | engelb@purdue.edu | Interim Head, Dept. of Ag. & Bio. Engr. |
| Daniel Ess | (765) 496-3977 | ess@purdue.edu | Precision Ag., Ag System Mgmt. |
| Jane Frankenberger | (765) 494-1194 | frankenb@purdue.edu | GIS and Water Quality |
| Carol Glotzbach | (765) 494-1174 FAX: (765) 496-1356 |
glotzbac@purdue.edu | Extension Secretary |