Pest & Crop Newsletter, Entomology Extension, Purdue University
- Identification of Six Alternative Winter Annual Weed Hosts for Soybean Cyst Nematode
- Weed Science Surveys IV: Field Surveys
![]()
Wheat, Aphids, Disease, and a Warm Fall – (Christian Krupke, John Obermeyer, and Larry Bledsoe)
- Aphids being reported in emerging wheat.
- Aphids can be carriers and vectors of barely yellow dwarf virus.
- Unseasonably warm fall temperatures may allow for longer than normal aphid infestation/feeding.
- Treating for aphids may pay in some circumstances.
To say that this fall has been warm would an understatement. In addition, long-range forecasts indicate that warmer than average temperatures are anticipated going forward. We know that warmer weather seems to almost uniformly favor insect pest populations. So as wheat emerges throughout the state and folks begin to observe aphids feeding on the seedlings, the hard questions begin: How long will the aphids feed and are they harming the wheat?
Aphids feed on wheat leaves by sucking plant juices with their straw-like mouthparts. Aphids have very little effect on the growing plant when moisture is available in the fall. This season, some wheat seed and/or seedlings may be in dry soil struggling to emerge and grow, making aphid competition for water even more critical. More importantly, aphids are vectors of barley yellow dwarf (BYD) virus. Aphids ingest the virus when they feed on diseased grasses (including volunteer grains) and then transmit the agent while feeding on wheat. Severity of BYD (remember that symptoms don’t show until next spring at green-up) is NOT dependent on aphid numbers. Not all aphids are carriers of the virus, so in a BYD year, a few aphids can do a lot of damage and vice versa. The level of BYD inoculum is an unknown that likely fluctuates from year to year.

Winged aphid feeding on wheat, vectoring BYD?
Aphids stay active, feeding and moving in the fall, as long as temperatures stay at 50ºF or above. After a killing freeze many aphids die and feeding drops off drastically. Some aphids manage to survive even the coldest of winters under clumps of wheat, though their feeding ceases. Therefore if temperature forecasts are correct, aphids may be feeding, multiplying and migrating to other fields much more through the months of October and November.
Aphid control with foliar insecticides to prevent BYD, especially with a potentially extended warm fall, is difficult at best. First, as already alluded to, as soon as aphids infest wheat they begin to feed. If a virus carrier, the disease transmission likely occurs shortly, almost immediately, after aphid feeding commences. If aphids are already present in a field, treating may prevent further spread within the field, but some infection will already have occurred. Secondly, the insecticide is only efficacious for for a relatively brief period. After a couple weeks (at most), the wheat is vulnerable to a new flush of migrating aphids. Finally, transmission of BYD virus also occurs in the late winter and early spring, after surviving aphids “awake” from their winter’s nap.
Wheat that is likely to benefit, not necessarily economically, from aphid control this fall:
- is under drought stress with aphids present,
- is a variety known to be susceptible to BYD with aphids present,
- is being grown for seed,
- is highly intensively managed, 100+ bu/A potentialv yield,
- was planted before the fly-free date.

Bird cherry-oat aphid nymphs beginning to feed
| Insecticides Labeled for Aphids in Wheat | |
| Product | Rate Per Acre |
| Baythroid2* (cyfluthrin) | 1.8-2.4 fl. oz. |
| Lannate SP* (methomyl) | 0.25 - 0.5 lb |
| Malathion 57 EC | 1-1.5 pts. |
| Mustang Max* (zeta-cypermethrin) | 3.2-4.0 fl. oz. |
| Penncap-M* (methyl parathion) | 2-3 pts. |
| Proaxis* (gamma-cyhalothrin) | 2.56-3.84 fl. oz. |
| Warrior* (lambda-cyhalothrin) | 2.56-3.84 fl. oz. |
| *Restricted use Product | |
Identification of Six Alternative Winter Annual Weed Hosts for Soybean Cyst Nematode– (Valerie A. Mock and Bill Johnson)
There are six winter annual weeds that have been identified as alternate hosts to soybean cyst nematode, and we have observed that SCN can reproduce in the field on purple deadnettle. Fields with these weed hosts may be increasing SCN population densities at a faster rate than fields without weed hosts. A recent study in Indiana found that known SCN weed hosts were prevalent in 93 percent of the fields surveyed (Creech and Johnson, 2006), indicating the possibility of a statewide increase in nematode population densities due to weeds. In Indiana SCN has been found in 82 of 92 counties (Faghihi et. al., 2006).
Most of these weeds can start to emerge during late august and September. So consider using this guide to scout fields and determine if you have these weeds present and if the density or future cropping plans would warrant fall treatments for winter annual weeds.
This purpose of this article is to the identifying characteristics of each of the six hosts. We will discuss them in the order of strongest to weakest host.
- Purple deadnettle (strong host)
- Henbit (strong host)
- Field pennycress (moderate host)
- Shepherd’s-purse (weak host)
- Small-flowered bittercress (weak host)
- Common chickweed (weak host)
Purple deadnettle and henbit are strong hosts and it can be difficult to distinguish the two weeds when they are small.
Purple Deadnettle (Lamium purpureum)
Leaves: Cotyledons have a white tip, and are oval with a notch where the petiole connects to the cotyledon. Leaves have prominent venation, resulting in a crinkled look. Leaves at the base of the stem are hairy and circular in shape. Leaves at the top of the stem are hairy and triangular in shape.
Stems: Square and greenish-purple in color. Stems tend to branch at the base of the plant and have hairs that point downward.
Flowers: Blooms are purple, and occur in upper leaves in whorls of 3 to 6. Purple deadnettle blooms between April and October.
Purple deadnettle cotyledons
Purple deadnettle
Henbit (Lamium amplexicaule)
Leaves: Cotyledons are oval, have a white tip, and notched where the cotyledon and petiole meet. Leaves are circular with rounded teeth along edges and slight venation which causes a crinkled look. Leaves have hair on their upper surfaces and hair along the veins on their lower surfaces. Leaves at the top of the stem wrap around the stem and are sessile.
Stems: Stems are square, tend to branch near the base of the plant, have hairs that point downward, and are green or purple.
Flowers: Flowers are purple to pink. Henbit can flower from March to November.
Field pennycress and shepherdspurse are weaker hosts than purple deadnettle and henbit. Much like purple deadnettle and henbit, these weeds can be difficult to distinguish from one another.
Henbit cotyledons
Henbit
Field Pennycress (Thlaspi arvense)
Leaves: Cotyledons are oval and have a bluish-green tinge with a long petiole. When the basal rosette forms, the leaf margin is wavy and slightly toothed. Leaves are egg-shaped, light green, and hairless. At maturity, no basal leaves are present. After bolting, leaves on the stem are oblong to lanceolate, have no petioles, have toothed leaf edges, and lobes that are pointed and clasp stem. Leaves emit a strong odor when disturbed.
Stems: Stems have no hairs. The leaves generally fall off the stem as the plant matures and can be branched in the top part of the stem.
Flowers: Flowers are white with four petals. Field pennycress flowers from April through June, and has a round seed pod (silicle) with a winged margin that is notched at the tip.
Field pennycress cotyledons
Field pennycress
Shepherd’s-purse (Capsella bursa-pastoris)
Leaves: Cotyledons have long petioles, and are egg shaped to round and narrower at the base. Young leaves are round and slightly hairy on surface with slightly toothed margins.
Generally, deeply lobed to deeply toothed leaves form around the 5th to 7th leaf and are dark green to silvery-gray.
Stems: Stems are unbranched and slender with gray hairs.
Flowers: White with four petals. Flowers from spring to early summer and sometimes in autumn. Seed pod is heart-shaped.
Shepherd's-purse cotyledons
Shepherd's-purse
Small-flowered bittercress (Cardamine parviflora)
Leaves: Basal leaves are deeply lobed, which gives the appearance of 3-6 pairs of leaflets with a rounded terminal leaflet.There are 4-10 stem leaves and no basal leaves at maturity. Leaves are generally hairless, but can be slightly hairy.
Stems: Branched with some leaves.
Flowers: Flowers are white with four petals. The seed capsule is a silique, which is long and narrow.

Small-flowered bittrcress cotyledons

Small-flowered bittercress
Common Chickweed (Stellaria media)
Leaves: Cotyledons are slender and ovate. Young leaves are opposite and round to egg-shaped with pointed tips. Mature leaves are oppositely arranged, light green, egg-shaped, pointed at the tips, and hairless. Hairy petioles occur on most leaves, but petioles are not present on the upper leaves.
Stems: Stems start to branch when five leaf pairs form. Stems are light green and generally smooth, but may have 1-2 rows of hairs.
Flowers: Flowers have five white petals that bloom from early spring to autumn.
It is important to reemphasize that these weeds can be found in a large percentage of no-till or reduced till fields in Indiana, and that SCN has been found in 82 of 92 counties in Indiana. We have conducted a number of field, greenhouse, and lab studies, funded by the Indiana Soybean Alliance and the USDA, to investigate the interaction between these winter weeds and SCN. We will discuss winter weed management in future articles, but for now, if you have any of these 6 hosts, and particularly the strong hosts, you should be concerned about management of these weeds and how that may impact soybean profitability.
Common chickweed cotyledons
Common chickweed
Glossary
basal rosette: Leaves radiating from the stem of the plant in a circular cluster at ground level.
cotyledon: The seed leaf.
leaflet: One subunit of a compound leaf.
petiole: The stalk between the stem and leaf blade.
sessile: Lacking a petiole.
silicle: Fruit of the Brassicaceae that is not much longer than wide (if at all).
silique: Fruit of the Brassicaceae that is an elongated capsule.
terminal leaflet: Occurs at the tip of the main compound leaf as a single subunit.
winter annual: Plant that germinates in late summer to early spring, flowers, produces seed in mid- to late spring, then dies.
References
Creech, J. E., and W. G. Johnson. 2006. Survey of broadleaf winter weeds in Indiana production fields infested with soybean cyst nematode (Heterodera glycines). Weed Technol. 20:1066-1075.
Bradley, K., and S. Hagood. Virginia Tech Weed Identification Guide. <http://ipm.ppws.vt.edu/weedindex.htm>. Accessed: 6/22/2007.
Faghihi, J., and V. R. Ferris. 2006. Soybean cyst nematode. Department of Entomology. Purdue University. <http://www.entm.purdue.edu/Entomology/ext/targets/e-series/EseriesPDF/E-210.pdf>. Accessed: 6/22/2007.
Uva, R.H., J.C. Neal, and J.M. DiTomaso. 1997. Weeds of the Northeast. Cornell University Press. South Korea. pp. 172-248.
Photo Sources: Kevin Bradley and Earl Creech
![]()
Weed Science Surveys IV: Field Surveys – (Glenn Nice, Bill Johnson, and Tom Bauman)
Yet other surveys are conducted by actively going out and sampling in fields. Two such surveys were to investigate the occurrence of glyphosate-resistant horseweed (Conyza Canadensis; referred to as marestail in Indiana) in the state of Indiana and the prevalence of stalk-boring insects in giant ragweed. In both of these surveys active sampling was done by taking samples in randomly or in selected fields.
Horseweed/Marestail. In 2002, two populations of glyphosate-resistant horseweed were identified in Jackson County, Indiana. The weed science teams of both Purdue University and The Ohio State University started a survey that would take three summers to complete. In Indiana, the sampling took the pattern of a target, the bulls eye being the South Eastern part of the state, where the first glyphosate-resistant population was identified (Figure 1). Sampling was set up as a randomized grid laid out before sampling began. Grid points were then manipulated to follow county roads; this was done so that sampling was accessible. This randomized approach was done to study the geographic distribution and frequency of resistance in the horseweed1. In 2003, 792 sites were survey and 389 samples were taken2. In 2004, 324 sites were surveyed and in 2005 214 sites were surveyed. At survey sites, when horseweed was present, seed heads were collected. Horseweed occurrence decreased moving north from 2003 to 2005.
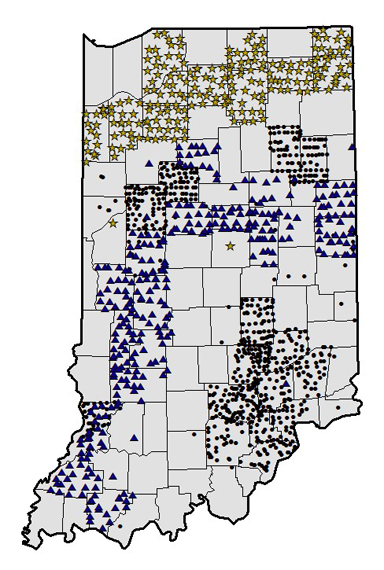
Figure 1. Horseweed (marestail) survey sites in Indiana. (2003 = circle; 2004 = triangle; and 2005 = star)
Collected seed was put though green house screens to identify any resistance to glyphosate. Seed was germinated and 2 to 3-inch plants were sprayed with a 2x rate to identify problematic populations. Susceptible plants showed injury 4 to 5 days after treatment and died 10 to 14 days after treatment. However, some plants recovered after initial injury and continued growing to produce seed. Today, populations have been identified that have low levels of resistance (2x to 4x) to glyphosate.
Presently, 2,4-D is being recommended for the control of glyphosate-resistant horseweed as a burndown application. To identify any possible issues with 2,4-D and horseweed control, 2,4-D was used in the screening process. Two populations of horseweed have been identified to have decreased susceptibility to 2,4-D3. Control ratings 21 days after treatment with 0.5 lb ai/A 2,4-D provided 69% and 62% control of two populations3.
Giant Ragweed and Stalk Boring Insects. Giant ragweed (Ambrosia trifida; sometimes referred to as horseweed in Indiana just to keep it interesting) is one of Indiana’s top ten problematic weeds. The suggestion that Indiana might be the “Giant Ragweed National Forest,” always gets nodes from people attending grower meetings. In 2003, the occurrence of giant ragweed escapes in Roundup Ready® systems started to raise concern about the control of giant ragweed. A survey was done in Indiana and Michigan to investigate the presence of stalk boring insects in escaped giant ragweed. This article will briefly discuss the Indiana data. Ten giant ragweed plants were selected in fields that had giant ragweed escapes in August and September. In August 2004, 71% of the plants had evidence of tunneling, 20% of plants had stalk-boring insects present4. In September 2005, 86% of the plants sampled had evidence of tunneling, 32% of the plants had stalk-boring insects present at sampling4. When insect identification was done, tunneling insects from the families Cerambycidae (long horn beetles), Curculionidae (weevils), Noctuidae (armyworm, etc), and Tortricidae (bell moths) were found (Table 1).
| Table 1. Percent of fields and escaped giant ragweed plants that had tunneling insects present in August (2004) and in August and September (2005). | |||||||||
| Family | Year | Region |
|||||||
Central |
Northeast |
Northwest |
Southwest |
||||||
Plants |
Fields |
Plants |
Fields |
Plants |
Fields |
Plants |
Fields |
||
| Cerambycidae | 2004 |
0 |
0 40 |
4 10 |
20 40 |
0 18 |
0 60 |
0 22 |
0 100 |
| Curculionidae | 2004 2005 |
8 14 |
80 60 |
4 4 |
40 40 |
14 2 |
80 20 |
10 6 |
40 60 |
| Noctuidae | 2004 2005 |
4 0 |
20 0 |
2 0 |
20 0 |
2 0 |
20 0 |
12 0 |
40 0 |
| Tortricidae | 2004 2005 |
4 18 |
40 80 |
8 10 |
60 80 |
4 12 |
40 60 |
0 0 |
0 0 |
| Adapted from Weed Technology 2007 21:526-531 | |||||||||
In a study conducted in the green house, 4-inch giant ragweed seedlings were inoculated with neonate European corn borer. Glyphosate was applied to 6 and 18-inch plants at three rates. In giant ragweed plants that did not get sprayed, European corn borer had no effect on giant ragweed dry weight5. When 6-inch plants were sprayed, tunneling did not have an effect on glyphosate efficacy. However, tunneling did have an effect on the low rate (0.5 lb ae/A) of glyphosate if giant ragweed was allowed to reach 18 inches before spraying (Table 2).
1. Davis, V.M., W.G. Johnson, and K.D. Gibson. 2004. Indiana glyphosate-resistant Horseweed (Conyza Canadensis) survey: current status. 2004 North Central Weed Science Proceedings 59:175.
2. Barnes, J., B. Johnson, and G. Nice. 2004. Update on the occurrence of glyphosate-resistant marestail/horseweed. <http://www.btny.purdue.edu/weedScience/2004/articles/updatemarestail04.pdf>.
3. Mock, V.A., V.M. Davis, J.E. Creech, and W.G. Johnson. 2005. Response of Selected Indiana Horseweed (Conyza Canadensis) Populations to 2,4-D. As presented at the NCWSS Annual Conference. <http://www.btny.purdue.edu/weedscience/PostSlide/horseweedMock05.pdf>.
4. Ott, E.J., C.K. Gerber, D.B. Harder, C.L. Sprague, and W.G. Johnson. 2007. Prevalence and influence of stalk-boring insects on glyphosate activity on Indiana and Michigan giant ragweed (Ambrosia trifida). Weed Technol. 21:526-531.
5. W.G. Johnson and E.J. Ott. 2004. Influence of stalk boring insects on glyphosate efficacy on giant ragweed. As presented at the NCWSS Annual Conference <http://www.btny.purdue.edu/weedscience/PostSlide/GIRWECBpos04.pdf>.
![]()
Soybean Rust Wrap-up - (Greg Shaner)
- Indiana growers got through another season with no rust.
Although soybean rust has spread extensively over the past 3 weeks, as far as we know it has not yet reached Indiana. Rust was found in far western Kentucky on September 21, and 5 days later was found just across the river in southern Illinois. Examination of the rust distribution map on the USDA PIPE website <http://www.sbrusa.net/> reveals a solid band of red counties (counties where rust has been detected) in eastern Arkansas that extends into southeast Missouri, southern Illinois, and western Kentucky. Rust was found in western Tennessee a couple of days ago. In all of these areas the crop is well past the stage at which rust can do any damage.
We are continuing to collect and examine leaf samples from fields in Indiana that still have green leaves, but such fields are becoming harder to find. Rust poses absolutely no threat at this time, but we want to determine whether infections have occurred in Indiana. Knowing the full extent of rust allows refinement of models that predict the spread of spores by wind and their deposition by rain.
This is the third season of soybean rust’s presence in the U.S., and the disease has not been anywhere near as widespread and destructive a problem as it has been in Brazil. Does this mean that soybean rust is not really a threat to U.S. soybean producers? We would like to think so, but the South has been under a drought the past 3 years. In many southern states, 2007 was the driest year since weather records have been kept. Rust does not do well in dry weather. This is probably why rust has spread so slowly in each of the past 3 seasons from overwintering sites in the Deep South, and only moved into the mid South and central states during mid to late September. By this time much of crop is well into seed development stages and the potential for damage to yield is low. If in some future year, southern states receive more rainfall in the spring and early summer, rust could develop quicker down there, and move into the Midwest earlier in the summer, so we can’t yet write rust off as a minor threat in the U.S.
![]()
2007 Ear Rot And Mycotoxin Survey Of The Indiana Corn Crop – (Charles Woloshuk)
- Hot and dry weather has raised question about the risk of wide spread mycotoxin contamination.
Many have asked whether there would be a mycotoxin problem in the Indiana corn because of the droughty and hot conditions we had this summer. Based on the early data from my annual ear rot and mycotoxin survey, I am fairly confident that we will not see widespread mycotoxin problems. Road trips from Rochester, IN to Bluffton, IN and then back to West Lafayette along the SR26 corridor, revealed plenty of stressed cornfields, but inspection of the ears did not reveal a significant amount of ear rot disease. I also made a trip to SW Indiana as far south as New Harmony. As a plant pathologist, I was a little disappointed because I found very little disease, although I did find a few fields with Diplodia ear rot and Fusarium ear rot, but the severity was low. Even in the sandy soils with corn plants only waist high, I could not find Aspergillus ear rot, the ear rot associated with aflatoxin contamination and often found under such stressed conditions.
As part of my annual ear rot and mycotoxin survey, I have received nearly 270 corn samples from Indiana’s Agricultural Statistics Service. Each sample contains five ears with the husks attached. I examined and rated the ears for ear rot, and samples with significant ear rot will be analyzed for mycotoxins. I have seen very little ear rot disease, and what I have seen has not been severe. Diplodia ear rot was observed in only three ears out of the 1350 ears that I have examined, far below the norm. Thirteen samples have sufficient Fusarium ear rot to warrant mycotoxin analysis for fumonisins. Most of these samples contain small ears with less than 200 kernels. Also, these samples do not come from any one region of Indiana.
Although the harvest period has been unseasonably hot, the good news is that the weather has remained dry. These conditions have allowed the grain to dry down, which should reduce the changes of more mold growth and mycotoxin accumulation.
![]()
Stored Grain Pest Management - (Linda Mason) - The harvest is over, the crop is ready to be stored and now you can relax knowing that you have produced quality grain. Right? WRONG! Harvest is the beginning of the storage period and implementing a stored-grain management program assures that grain quality is maintained.
Stored-grain management integrates practices such as sanitation, application of grain protectants, temperature and aeration management, and sampling to assure that grain quality remains high.
Thorough inspection and cleaning of bins prior to filling can eliminate many problems. Make needed repairs to walls and roofs to prevent entry of pests and moisture. Bin walls, floor, and subfloor should be cleaned to remove any grain residue that could serve as inoculum for insect and mold infestations. Don’t forget to clean up grain that has spilled around the exterior of the bins and loading areas. Use herbicides to remove all grass or weeds around the storage structures to prevent harborage for rodents and insect pests. Remove all spills as soon as they happen so that insects and rodents are not attracted to the storage facility.
Sanitation is the most effective and economical management practice to prevent pest infestation. It is usually less expensive to be proactive than reactive when it comes to pests. After cleaning, an approved residual insecticide may be applied to the outside and inside bin walls, and floors. Residual insecticides are effective in reducing pest risk by limiting population growth.
It is recommended that a grain protectant be applied to grain that will be in storage for one or more years, and for all grain that will be stored at ambient temperatures above 55°F. This is especially true in southern or tropical climates where warmer grain temperatures encourage insect growth year-round. Grain protectants are insecticides registered for application to whole grain to protect against insect infestations while the grain is in storage. Do not apply protectants before high temperature drying because extreme heat will result in reduced the effectiveness and residual time of most insecticides.
Residual insecticides are often applied to grain while it is being augured into the bin. However, surface treatments (top dressing) post binning can be just as effective. Be careful not to exceed legal tolerances if grain was treated during the binning process. Remember to level the grain surface; this will improve airflow through the bin, allowing for more uniform temperatures. Coring a bin is also important in preventative pest management. A core of fines creates non-uniform air flow and as a result, non-uniform temperatures. It also provides a food source for a majority of storage pests. Correctly aerating and managing stored grain minimizes quality deterioration.
Grain temperature, moisture levels, and insect activity should be periodically monitored throughout the storage period to prevent spoilage. When grain temperatures are below 55°F, monitor for insects on a monthly basis. As the grain warm, increase pest monitoring to once a week. Pheromone baited flight traps allow you to monitor moth populations, while grain probe traps monitor beetle populations. When placed outside the bin you can monitor pest pressure in your facility. Traps located within the bin act as an early warning to pest problems. Make sure to examine trap data on a regular basis, it is not always the absolute number of insects you catch but the trend that is important. A steady increase in the population indicates a growing problem.
![]()