Pest & Crop Newsletter
|
||||||||||||||||||||||||||||||||||||||
Soybean Aphid Numbers Increasing in Northen Indiana– (John Obermeyer and Larry Bledsoe)
Soybean plants may be looking yellow and puny, but the soybean aphid doesn’t seem to mind. Research fields in northern Indiana, along U.S 30, have had a dramatic increase in aphid numbers over the past week. In these fields, 100% of the plants sampled had on average 14-30 aphids/plant. Winged aphids were present, indicating that they are moving to other locations. This population surge is not being seen in monitored fields further south, near Lafayette. The University of Illinois is reporting threatening populations in the state’s northern counties. Presently the lack of beneficial predators, parasites, and pathogens in these infested fields has been disappointing. The most common predator that Purdue researchers are reporting is the minute pirate bug (Orius tristicolor). This has been the most consistent natural enemy found in soybean fields, even before soybean aphid was known to occur in the Midwest. The minute pirate bug as the name implies, is very small. It is one of the first predators to appear in early growing soybean plants and is thought to keep most early invading aphids in check. The much larger and obvious Asian lady beetle has been very low in numbers this season. Reports from heavily infested fields in northern Illinois are noting an obvious decline in this beetle’s population from previous seasons. Nobody knows for sure when is the best time to treat for soybean aphid, a.k.a., economic threshold. There has been much discussion about aphids/plant and aphids/trifoliolate leaves, etc., but no hard and fast rules. At this time, our recommendation is that commercial soybean fields should not be treated until symptoms become evident. When high stress areas of a field are beginning to yellow (e.g., low potassium levels, sandy soils, soybean cyst nematode) and aphids are quite evident (live aphids and sticky plants from honeydew) as you walk through the field, then treatment may be justified. It is extremely important to assess aphid-infested fields for beneficial organisms before management decisions are made. Treating soybean with an insecticide for the remainder of the season may tip the balance in the favor of soybean aphid. In other words, natural enemies recover slowly from broad-spectrum insecticides compared to aphids. In general, toxic levels of insecticide are absorbed by ingestion (eating treated leaves) and/or contact (walking over treated areas). Aphids are sucking insects and ingest only internal plant fluids. As well, except for mature females, they are relatively stationary on the bottom sides of leaves; obviously a difficult location to get thorough coverage. A very important note is that surviving aphids can repopulate fields at break-neck speed, certainly outpacing natural enemies.
Time to Monitor for Western Corn Rootworm Beetles in Soybean– (John Obermeyer and Larry Bledsoe)
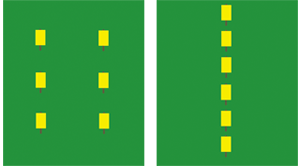
Western corn rootworm beetle numbers seem to be much lower this year. This would be an excellent year to implement a weekly scouting program to determine the risk of rootworm larval feeding to next year’s corn. Why is there rootworm damage in corn following soybean? Portions of northern Indiana have been affected by a dramatic change in western corn rootworm (WCR) beetle behavior. Previously, WCR adults laid eggs primarily in cornfields, but now variant WCR are laying large numbers of eggs in soybean fields, resulting in economic root damage to corn the following growing season. This behavioral change has virtually eliminated the benefit of crop rotation as a rootworm management tactic in the most severely affected regions of the problem area and has resulted in routine applications of soil insecticides to most cornfields. What can be done to reduce unnecessary insecticide applications? How should traps be used to monitor WCR beetles in soybean? Beginning no later than July 24, place 6 PheroconÆ AM (unbaited) yellow sticky traps (sticky surface out) on stakes slightly above canopy level and distributed throughout a soybean field, keeping at least 100 feet away from field edges and/or waterways. Consider that large fields (>60 acres) with variable soil types, weed control, etc., will need more traps to improve estimates of rootworm abundance. Divide field into representative units if necessary. Remove soybean plants around the stakes to prevent leaves from sticking to the traps. For ease of collecting traps in drilled soybean, consider placement along wheel tracks, skipped rows, etc. Each week for 6 weeks, or until the beetle threshold is reached, remove the traps, and place new ones just above the soybean canopy. Count and record the number of rootworm beetles on each trap. To determine the average number of beetles/trap/day, add the numbers for the 6 traps in each field, divide that number by 6, and then divide by the number of days the traps have been in the field. Although a 7-day sampling period is preferred, be sure to divide by the actual number of days the traps were in the field to determine the average. When do trap counts indicate the need for a management tactic? If the Pherocon® AM traps in soybean fields average 5 or more beetles/trap/day during any trapping week, some management tactic should be implemented for WCR larval control in next year’s corn. Management options include: 1) rotation to a crop other than corn or 2) using a rootworm insecticide. In research fields where at least 5 WCR beetles/trap/day in soybean were observed, >95% of the cornfields reached economic root damage the following year. Do not use a single trapped field to estimate rootworm abundance in surrounding fields. Where can I get the traps? Pherocon® AM yellow sticky traps can be purchased from several distributors. Two possible sources are: Gempler’s (800-382-8473) and Great Lakes IPM (800-235-0285). This listing is not all inclusive, nor an endorsement by Purdue University. The manufacturer of the Pherocon® AM yellow sticky trap is Trece Inc. (831-758-0204).
Where can I get more WCR information? WCR life history, damage, sampling methods, and management guidelines are available in the Field Crops Pest Management Manual (IPM-1). Updates of Indiana’s risk areas and control products for this pest are presented in the publication E-49 Managing Corn Rootworms located at: <http://www.entm.purdue.edu/entomology/ext/targets/e-series/e-list.htm>. For these and other publications, call Purdue Extension at 765-494-8491
Click for Table
|
||||||||||||||||||||||||||||||||||||||
Indentifying Glyphosate-Resistant Marestail/Horesweed in the Field- (Jeff Barnes & Bill Johnson) Herbicide-resistance is not a new issue in Indiana and was first documented in 1980 when populations of redroot pigweed and lambsquarters were identified that were resistant to atrazine. Since that time nine additional weed biotypes of weeds have been identified as herbicide-resistant. The most recent weed to gain this designation is marestail [aka horseweed (Conyza Canadensis)] that is resistant to glyphosate. Even though Indiana producers have potentially had to deal with herbicide-resistant weeds for more than 20 years, the number of infested fields and acres has occurred in relatively isolated instances. Herbicide-resistant weeds were believed to have only infested 17,000 of Indiana’s 13 million acres of cropland in 2002. The relatively slow development of herbicide resistant weeds may have lulled many of us to anticipate that as resistant weeds were identified there would be plenty of time to develop effective management plans to prevent the widespread distribution of “super” weeds. Every now and then we all get a wake-up call. The wake-up call for weed scientists and crop producers alike has been glyphosate-resistant marestail. While marestail control with glyphosate has always been tricky if allowed to get to large, this winter annual weed could usually be effectively controlled by paying attention to plant size and targeting small horseweed with burndown glyphosate applications. Glyphosate-resistant marestail first arrived on the scene in Delaware in 2000 in continuous soybean fields that had been treated with only glyphosate for three years. Extension personnel in Delaware now estimate that 100,000 of the states 560,000 acres of cropland are infected with glyphosate-resistant marestail. A similar bad scenario has been playing out in Tennessee where one field was documented as resistant in 2000. In 2002, Extension specialists estimated that more than 400,000 of cropland were infested with this rapidly spreading problem. Fields containing marestail are now generally assumed to have some level of resistance within the marestail population forcing additional expense for burndown weed control alternatives. Nine other states including Indiana have either confirmed or suspect the presence of glyphosate-resistant marestail.
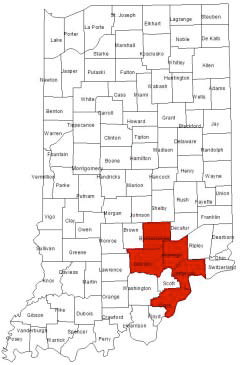
Indiana’s problem has been isolated to the southern portion of Indiana and was first reported in 2002 (Figure 1). Two populations of marestail were collected from fields in Jackson County in the fall of 2002 and were subsequently tested for glyphosate-resistance in the greenhouse. Both Jackson County populations were tolerant to glyphosate applied at rates as high as 4x the normal use rate (see <www.btny.purdue.edu/weedscience/2003/ This fall weed science personnel are going to be conducting an extensive survey to get a handle on the distribution of glyphosate-resistant marestail in Indiana. We will also be able to develop area-specific burndown and in-crop weed control recommendations based upon the potential risk of discovering the resistant weed in an area. One important goal is to isolate the problem as much as possible before it explodes over a large acreage in Indiana as it has done in other states. This goal will be easier to achieve once we can gain some perspective on how widespread the problem is at the moment. A secondary objective is to screen the collected populations for possible resistance to other herbicide chemistries. Assistance is needed in identifying fields that potentially contain glyphosate-resistant marestail. Unfortunately there is no proven method to determine if the marestail in a particular field is resistant to glyphosate prior to actually making a herbicide application. Knowing a field’s herbicide and production history will not be totally adequate in determining if a field has glyphosate-resistant marestail. The seed of marestail is spread by the wind, which is one reason for the rapid acreage expansion within other states. Research in Pennsylvania and Argentina have shown that marestail seed can disperse up to a 1/4 mile in a mild wind of 10 MPH. Just think how far the seed could move on a blustery fall day when the winds are substantially higher. Pinpointing Fields With Glyphosate-Resistant Marestail The only sure way to tell if marestail in a field is glyphosate-resistant is to spray the field and see if marestail dies. Glyphosate-susceptable and –resistant marestail are morphologically the same and is believed to follow the same emergence and growth patterns. Unfortunately if the marestail does not die it might be to late to implement alternate control strategies without affecting crop production. Applications of 2,4-D with glyphosate can be effective as a second treatment to control the “missed” marestail but attention must be paid to recropping intervals particularly to soybean, which for most 2,4-D products is 30 days prior to planting. When examining a field to determine if the missed marestail is potentially glyphosate-resistant, follow a few key guidelines listed below.
While these guidelines are not perfect by any means, hopefully they will help to identify fields that potentially have resistant populations of marestail. As your driving down the road right now, marestail can be seen along many of the roadsides and even in fields sticking above the soybean crop. Marestail will often grow to a height of 2 to 3 ft and is substantially taller than most of the soybean crop at this time. As fields are identified in which marestail control has failed this season please contact us using the information listed below. We are hoping to identify as many fields as possible that have potential resistant populations and map those fields to develop a database on the location of these fields. Later this summer and early fall we will come back into those fields and collect seed samples which will be tested for resistance in greenhouse studies. We are also looking for a few fields in which the producer will be willing to allow us to conduct research trials this fall and next summer to further develop recommendations for short-and long-term control of glyphosate-resistant horseweed. Your assistance will be invaluable to the success of this project. If there are any questions or comments that you would like to make, please feel free to contact us at the numbers below. If you need to report a potentially resistant marestail field or would like us to visit fields with you then please free to contact us as well. Contact Information Jeff Barnes Bill Johnson Glenn Nice
|
||||||||||||||||||||||||||||||||||||||
Wheat Diseases- (Andreas Westphal and Charles Woloshuk)
Previous articles in Pest&Crop (nos. 10, 12, 14) have discussed the risk and the actual occurrence of wheat head scab in Indiana, a disease caused by Fusarium graminearum. The fungus is easy to detect when a light pinkish color is observed on the wheat heads or kernels. The continued rain over much of Indiana has made it difficult to harvest wheat, increasing the risk for low quality grain and contamination with the mycotoxin, deoxynivalenol (DON). The wheat scab infections occur right at flowering, and the most severely diseased kernels do not fill, but remain small and shriveled. These kernels will lower the bushel weight and will contain the highest levels of DON. Adjustments to the harvest combine can lower the amount of these kernels in the harvested grain. With the continuous wet conditions, there is a growing concern about kernels that appear to be healthy but are in fact colonized by the fungus. With grain still in the field under the current moist and warm conditions, the fungus can continue to grow in these kernels and the potential for DON contamination increases. To lower the risk of DON contamination, the wheat should be harvest as soon as possible and dried below 13% moisture content to stop fungal growth. While drying will not reduce the DON level, it will prevent the production of more DON. Mycotoxins such as DON are compounds that are deleterious to animal and human health. The U.S. Food and Drug Administration has established advisory levels for DON in food and feed (Pest&Crop no. 14). The DON content can be determined using a variety of test kits (see: BP-47 “Mycotoxins and Mycotoxin Test Kits” at <http://www.agcom.purdue.edu/AgCom/Pubs/BP/BP-47.html>).
|
||||||||||||||||||||||||||||||||||||||
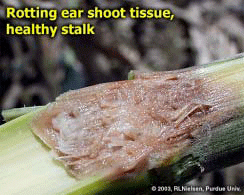
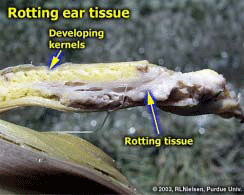
Bacterial Ear Rot in Corn Due to Flooding- (Bob Nielsen) The Great Flood of ’03 will be remembered for the crop devastation caused by the flooding of the Wabash River and many of its tributaries. As the flood waters recede, the totality of crop death is immediately evident in those areas where crops were totally submerged for a period of days. Less obvious is the damage to plants on the higher elevations within the flood plain that were only partially submerged, particularly those fields where pollination was in progress or that were in the early grain filling period following pollination. These plants withstood the onslaught of flood waters that rose to heights above the ear but quickly receded with little to no major structural damage to the plants. Unfortunately, these survivors along the fringes of the major flooding may have won the battle, but may lose the war because of the potential for the development of bacterial ear rot as a consequence of the exposure of the immature ears to the muddy flood waters. The following images illustrate the occurrence of bacterial ear rot in a corn field along the Wabash River in Vermillion County, Indiana. The field was adjacent to one that was totally destroyed by flood waters, but which itself had been briefly immersed up to and just beyond the ear shoots. The corn plants themselves were still green and technically alive, but the husk leaves were a discolored, slimy, soft, and smelly mess; especially at their basal ends near the point of attachment to the stalk node. The immature ears also exhibited varying degrees of gray, slimy, and soft rotting tissue. The odor associated with this slimy mess of rotting plant tissue reminded me of fermenting corn silage. Bacterial stalk rots in corn are more commonly reported than are bacterial ear rots; often developing under warm & humid conditions or in conjunction with pivot/sprinker irrigation systems (Shaner, 1998; Stack, 2002). While less common, bacterial ear rots have been reported before in Indiana following similar flooding conditions (Nielsen & Ruhl, 1998). Bacterial ear rot is caused by one of several species of soft rot bacteria that live as saprophytes on plant debris in the soil. During periods of high rainfall, flooding, overhead irrigation, or poor drainage; bacteria are splashed onto plants and infect susceptible tissue. The bacteria normally enter the plant through leaf stomates or wounds on leaves or stalks. While there is no remedy for this flood-related problem, growers should nonetheless scout areas of field that were partially immersed by the outer reaches of flooding rivers and creeks to more accurately assess the full extent of the flood damage to their corn crops. Related References Nielsen, RL (Bob) and Gail Ruhl. 1998. Bacterial Ear Rot in Flooded Corn. Purdue Univ. Coop. Ext. Service. Available online at <http://www.kingcorn.org/news/articles.98/ Shaner, Greg. 1998. Bacterial Stalk Rot. Pest & Crop Newsletter (17 July 1998). Purdue Univ. Coop. Ext. Service. Available online at <http://www.entm.purdue.edu/entomology/ext/ Stack, Jim. 2002. Bacterial Stalk Rot. Univ. of Nebraska Coop. Ext. Service. Available online at <http://pdc.unl.edu/corn/bacterialstalkrot/>. [URL verified 7/18/03]. For other Corny News Network articles, browse through the CNN Archives at <http://www.kingcorn.org/news/index-cnn.html>. For other information about corn, take a look at the Corn Growers’ Guidebook at <http://www.kingcorn.org>.
Struggling Soybeans- (Ellsworth Christmas- Reprinted with permission from AgAnswers, Steve Leer, writer)
Indiana soybeans have suffered a beating at the hands of Mother Nature this spring and summer. It’s no wonder, then, that the crop looks down for the count, said Ellsworth Christmas, Purdue University Cooperative Extension Service soybean specialist. Many soybean fields in central and northern Indiana that weren’t washed away by floods are stunted and pale from multiple storms, standing water and fluctuating temperatures, Christmas said. In southern Indiana, excessive spring precipitation pushed back soybean planting, reducing yield potential, he said. “Right now the big concerns are related to the color of the crop and the fact that it is not growing,” Christmas said. “That’s all related directly to waterlogged or saturated soils. Any time you have saturated soils the nodules are not producing adequate nitrogen for the plant. “Couple this with the fact that we’ve had overcast days, and the plants were not producing a lot of photosynthates to send down to that root system to support the roots as well as the nodules. However, should the soils dry out and the nodules become either more active or re-established, then we’ll see the plants start to darken in color and look quite normal.” For the moment, the crop continues to decline. As of Sunday (7/20), 49 percent of Hoosier soybean acres were rated “good” or “excellent,” down 2 percent from one week earlier and off 8 percent since July 6, according to the Purdue-based Indiana Agricultural Statistics Service (IASS). The IASS rated 19 percent of acres “poor” or “very poor” on July 20, up 8 percent in two weeks. Plant development also has slipped, the IASS reported. Thirty-six percent of the soybean acreage was blooming by July 20, up 1 percent from the same period in 2002 but well off the five-year average of 63 percent. Only 5 percent of soybean acres were setting pods by Sunday, compared with 18 percent for the five-year average. Indiana farmers planted about 5.4 million acres of soybeans this year, down 7 percent from 2002. Christmas said soilborne diseases could further damage an already fragile crop. Soybean fields in northern Indiana are especially vulnerable, he said. “A couple of things we need to be on the lookout for are diseases that can be triggered by these weather conditions,” he said. “One of those is Sudden Death Syndrome, particularly if those plants were under a lot of stress early and infection occurred. If we get rainy conditions or saturated soils during early pod development, it could trigger the toxic phase of Sudden Death Syndrome. “The other disease, which most likely will be in northern Indiana, is Sclerotinia, or what we call white mold. Again, we have wet conditions, high humidity in the canopy, relatively cool nighttime temperatures and flowers on the plant. This all is very conducive to white mold infection.” Sudden Death Syndrome (SDS) can ruin a soybean crop. The SDS fungus, which favors wet field conditions, produces small yellow blotches on soybean leaves. The plant tissue within the infected area becomes brown and dies, impairing the plant’s grain-making ability. White mold attacks the soybean plant’s stem, covering it with a light-colored fluffy growth. These lesions cause premature plant death. Farmers in southern Indiana counties struggled to get soybeans planted by May 20, the ending date for maximum yield potential. Most soybean acres in the region were seeded around mid-June or later, Christmas said. “Yield potential on late-planted beans is going to be lower. We’ll see that happen this year in the southern third of the state,” he said. “The one good thing about it is we’ll probably see a lower incidence of Sudden Death Syndrome in southern Indiana than we normally see.” Root rot diseases are surprisingly absent from the late-planted crop, Christmas said. “Let’s hope that we have good growing conditions the remainder of the season and get adequate moisture during August and early September, to fill the pods on the late-planted beans in southern Indiana,” he said. At this point, farmers can do little to improve their soybean crops other than control weeds, Christmas said. He advised against applying nitrogen — even to plants starved for the nutrient. “It’s a waste of money because the plants prefer the nitrogen when it’s applied either as a fertilizer or when it’s available in the soil as organic material that breaks down,” he said. “If you apply nitrogen you can make the plant look better, but it’s not going to do you any good in terms of yield.”
Testing Corn Leaf Tissue- Is It Important?- (Maurice Watson, Ohio State University)
Plant tissue analysis is a diagnostic tool that often has been overlooked by growers. Determining the concentration of nutrients that is actually in the corn plant can provide important information about problem areas and management practices for corn production. Plant analysis can be used to sdiagnose nutritional problems that may exist in certain areas of the field, or it can be used to monitor the crop to evaluate the overall nutrient status of the crop. Using plant analysis to evaluate your corn crop is very useful. Correct sampling is important to ensure useful analytical data. The ear leaf should be sampled for testing when the corn is in the initial silk stage of growth. The nutrient concentrations in the ear leaf have been shown to be most highly correlated with corn yield. Approximately 10-20 leaf samples should be taken randomly across each acre of the field. If there is an area of the field that is suspect be sure to test that area separately. Do not sample dead or diseased plants. Be sure to handle the plant tissue after sampling in accordance with the instructions provided with the sample kit.
|
||||||||||||||||||||||||||||||||||||||