Pest & Crop Newsletter
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Alfalfa Weevil Management Guidelines and Control Products - (John Obermeyer, Rich Edwards, and Larry Bledsoe)
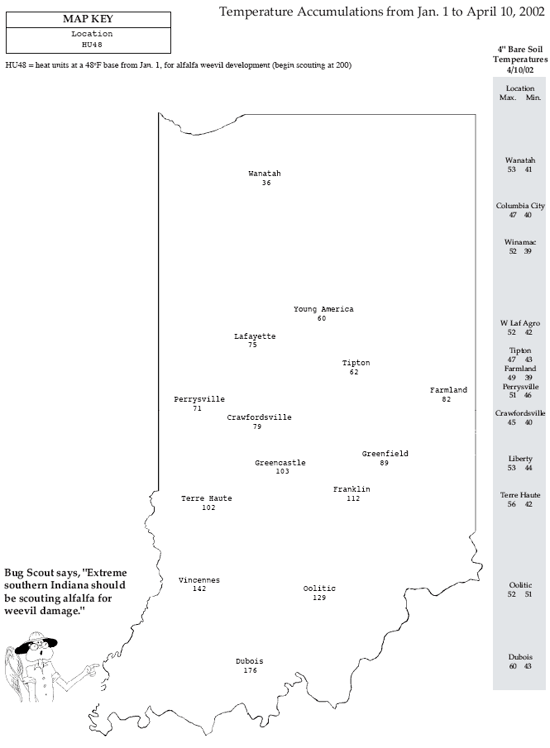
Last week’s sampling results and pest manager reports from southern counties point to the possibility that alfalfa weevil damage my equal or even exceed last year’s tremendous levels. As mentioned in last week’s Pest&Crop, their development and damage is ahead of the heat unit model that normally accurately predicts weevil activity. This is especially true on south facing slopes or fields with sandier soils. The management guidelines listed below should be used as a guide in determining when alfalfa weevil should be controlled in southern Indiana. The times for sampling and the need for and timing of controls are based on accumulated heat units (HU) at a base temperature of 48∞F and percentage tip feeding. Refer to HU information in each week’s Pest&Crop “Weather Update.” This HU information will help one determine when management steps should be taken.
What About Seed Attacking Insects? – (John Obermeyer, Rich Edwards, and Larry Bledsoe)
Most of our attention to soil insects is given to corn rootworm, what about those other critters? Wireworms, grubs, maggots and seedcorn beetles occasionally damage seed and seedlings. Obviously, the longer that germination is delayed, the greater the chance for insect damage to occur. How about the seed that will be planted during the next window of opportunity, should it receive a seed treatment to protect from these occasional pests? The following discussion is for these other soil insects, NOT ROOTWORM. Planting in fields with less than adequate drainage, in set-aside acreage (such as CRP land), or fields with high crop residue or where high rates of manure have been applied, the use of a seed protectant may be a good investment against seed attacking insects. Seed protection will be critical if our cool weather pattern continues and soil temperatures remain at less than ideal levels for rapid seed germination and plant growth. Planter box seed treatments, such as Kernel Guard Supreme and KickStart VP are registered for both corn and soybean. The insecticide permethrin, same active ingredient in the foliar insecticides Ambush and Pounce, in these seed treatments should provide adequate control of seed maggots and beetles. In limited trials, permethrin has shown some protection from wireworms. Because seed treatments do not protect the plant once it sprouts, there is no control of white grubs, cutworms, rootworms, or high populations of wireworms. Pre-applied insecticide seed treatments are now available for corn producers. Industry and university trials have shown some promising results with Gaucho, Prescribe, and ProShield against wireworms and seedcorn maggot. As well, the systemic activity of Gaucho and Prescribe provides some early suppression/control of corn flea beetle. Certainly the biggest question for producers and researchers is how effective these products are against white grubs. Limited trials have shown a mixed bag of results. Most likely there will be some suppression of grubs, but not control. Where rootworm soil insecticides are applied at planting, the use of a planter box or pre-applied seed treatment is not necessary.
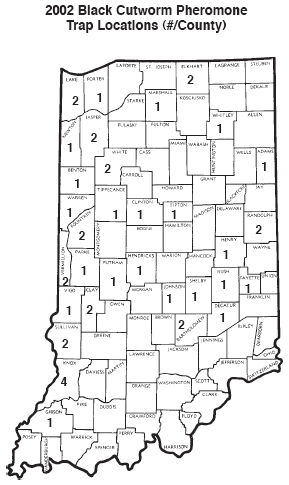
Black Cutworm, They're Here – (John Obermeyer, Rich Edwards, and Larry Bledsoe)
Several black cutworm intensive captures, 9 or more moths caught over a 2-nights, have been captured during the week of April 8 (see “Black Cutworm Adult Pheromone Trap Report” and “2002 Black Cutworm Pheromone Trap Locations”). This correlated well with the warmer temperatures from the Southwest that swept across the Midwest and brought black cutworm moths from Mexico and Texas. The timing of their arrival is normal, the moth flights of mid to later April are usually the ones we carefully monitor. New arriving moths are looking for the perfect place, i.e., winter annuals, for egg laying. Fields that are now covered in chickweed, mustards, etc. are at highest risk for cutworm damage. Remember, corn and soybean are not the black cutworm’s food of choice. These are normally the only plants remaining by the time larvae have hatched and weeds are dead. Research has shown that cutworm larvae starve if weeds are destroyed 2-3 weeks before corn emergence. This says something for early burn-down herbicides in the management of this pest. Look for updated pheromone trap captures and heat unit tracking of cutworm development in future issues of the Pest&Crop.
Black Cutworm Adult Pheromone Trap Report
Southwestern Corn Borer Spring Survey – (Ric Bessin, Lee Townsend, Wayne Mattingly, and Mike Smith, University of Kentucky) Southwestern corn borers spend the winter as larvae in galleries at the base of corn stalks. Stubble in cornfields can be checked during early spring for damaged plants and surviving borers. This can provide an indication of what the first generation may be like for 2002. A survey of southwestern corn borer damage and larval survival was conducted in Caldwell, Daviess and Henderson counties on March 14 and 15. These counties were selected because of the past infestation history. The purpose was to estimate the extent of SWCB damage, as evidenced by basal stalk girdling. In addition, we wanted to estimate the survival of the overwintering larvae in the crowns of these damaged plants. In each county, three to five non-Bt corn fields were evaluated. Within each field, 10 to 12 groups of 10 plants were examined for girdling damage and presence of live SWCB larvae. An additional 50 damaged plants were examined for the presence of live SWCB larvae. This is the fourth year that we have conducted such a survey. In comparison to the previous winters, we had the lowest levels of girdled plants and survival of overwintering larvae. Fewer girdled stalks were to be expected, because planting conditions in April 2001 were excellent. This allowed growers to get their corn crop in the ground on time and enabled early harvest. Delayed harvest allows SWCB time to migrate to the bottom of the stalk and girdle the plant. Early planted corn may also be less attractive for lateseason egg laying. Observed levels of survival in the girdled crowns was surprising. Survival this spring is less than what would have expected considering the relatively mild winter. Of the girdled crowns sampled this spring, a large proportion had evidence of bird activity with the larva having been removed. Relatively few crowns had dead larva remaining in the overwintering chamber. The number of live SWCB larvae per stalk is a small fraction of what we estimated in other years. This survey indicates that there are potentially fewer SWCB moths to begin the season as compared with the past three years.
Keep in mind that overwintering survival is just one of the variables that will, in part, determine the potential for SWCB problems in 2002. Historically, the date of planting of individual fields has been a key variable contributing to the potential for late season SWCB damage. Although early season numbers seem to be very low, favorable conditions, may allow SWCB numbers to rebound by the second and third generations. Typically, fields planted after May 10 have an increased potential for this type of damage. What we can conclude:
Reprinted from Kentucky Pest News, Number 943, March 18, 2002.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Soil, So What?- (Glenn Nice and Thomas Bauman) What many of us call “dirt” is a diverse and complex medium involving physical and chemical processes driven in part by a multitude of living organisms. As could be expected, the soil is a highly variable environment that is effected by its history, weather patterns, chemical and mineral makeup. Soils are also dynamic, meaning that they are in a constant state of change. Components are added and lost through time. With this diverse medium compounded with the diversity found in herbicide make up, it is no surprise that herbicides may react in many different ways when applied to soils. Below are some of the interactions that might take place with a herbicide and the soil. These interactions may lead to persistence problems or a decrease in herbicide efficacy. Adsorption vs. Absorption In both cases, the herbicide can be taken out of the soil solution decreasing herbicide activity. Further more, in both cases the herbicide can be put back into the soil solution. The difference between the two terms is that adsorption refers to the collection of the herbicide on the soil particle surface. Absorption refers to the taking in of the herbicide into plants or microbes. Adsorption is one of the most important ways in which a soil applied herbicide is made unavailable to do its job. The attraction is fueled by the electrostatic charges found on the soil particles. Depending on the charge of the herbicide molecule in the soil, adsorption can occur on either the organic particles or the inorganic particles. This is why some herbicides have higher use rates or are not recommended for soils with high organic matter. Ion exchange can also lead to the adsorption of an herbicide’s active ingredient. Dry soils may have a higher rate of adsorption than wetter soils. The measurement Kd represents a herbicides inclination to adsorb to a soil. This is the ratio of herbicide bound to a soil and the amount that is still in solution. Kd is often used in models to predict a herbicide’s potential to movement through soil. The greater the Kd the greater the tendency to bind to a soil. Note glyphosate’s high Kd value (Table 1). This gives some explanation as to why glyphosate is not to be considered to have any residual soil activity. For a list of Kd values, see the Herbicide Handbook released by the Weed Science Society of America <www.wssa.net>.
Absorption of herbicides into plants and microorganisms is another way in which a herbicide can be rendered benign. Once taken up by the organism, it is temporarily out of the soil medium. In some cases, a portion of the herbicide is not changed in the living organism and can be released back into the soil. The release of an adsorbed or absorbed herbicides is called desorption. This can be good in the sense that the herbicide can have some residual activity. However, it can be bad in the case of persistence (carry over). Leaching Leaching is the movement of the herbicide while in the water solution through the soil. A small amount of movement is needed to get the herbicide in the zone of germination. This is obtained by a small light rainfall event or with some herbicides (such as Treflan) incorporation. However, too much movement is a cause for concern. Leaching is most associated as being a problem due to groundwater issues, but there are other problems that arise due to leaching. Movement of the herbicide from the zone of germination results in reduced weed control. The lateral movement of a herbicide can increase accumulation in the seed furrow resulting in an injurious concentration. In dry conditions, the upward movement of soil moisture can bring the herbicide to the soil surface resulting in increased evaporation. Some of the herbicides that have high leaching potentials are atrazine; dicamba; imazaquin; and picloram. However, some of the herbicides that have moderate leaching potentials are clomozone; linuron; pendamethalin; and trifluralin. Several factors have an effect on a herbicide’s likely hood to leach. Soil texture and permeability influence can influence leaching. Herbicide movement through coarse textured soils is one of the reasons atrazine products are not recommended for sandy soils with shallow water tables for fear of drinking water contamination. As might be expected the volume of water flow and direction (up or down) have a strong influence on a herbicides potential to leach. If the herbicide will be adsorbed to the soil and its water solubility also has a large influence leaching. Chemical Reactions There are several reactions that can occur between a herbicide and the elements of the soil. Oxidation-reduction reactions can create electrically charged molecules which in turn will be made unusable by the plant or adsorb to oppositely charged soil elements. These oxidation-reduction reactions involve the donation of an electron either to or from the herbicide. The charged herbicide particles are then likely to form bonds with other soil components. The bonds formed with calcium (in a high calcium soil) can form water insoluble salts, making the herbicide unavailable to control weeds. Also, complexes can be formed with some of the metals found in the soil, such as cobalt, copper, and iron. These complexes are also useless as a herbicide. One of the main ways in which sulfylureas are broken down is through hydrolysis. Herbicides molecules can react with water in hydrolysis. Herbicide molecules break and ionic components (H+ or OH-) of water bond to the broken molecules. The new molecules formed generally don’t have the herbicidal activity. Microbial Degradation The soil is a living microcosm including bacteria, fungi, algae, nematodes, protozoa, worms, and insects. However, in the breakdown of herbicides, it is predominantly the task of bacteria and fungi. In their constant search for food, microorganisms will take in organic compounds, including herbicides. Like our own digestive track, bacteria and fungi produce a multitude of enzymes to break down complex molecules. It is these enzymes that degrade herbicides. W. P. Anderson list in Weed Science Principles and Applications (1996) some of the alterations that occur due to enzymatic reactions; dehalogenation; dealkylation; amide or ester hydrolysis; beta-oxidation; ring hydroxylation; ring cleavage; and reduction of nitro groups under anaerobic conditions. The rate of herbicide degradation is directly related to the population numbers, rate of metabolism, other available nutrients, and the type of herbicide present. Factors that affect the microorganisms are soil moisture, temperature, oxygen (aeration), mineral nutrient supply, organic matter content, and soil pH. Temperatures between 75 and 90°F are generally optimum for microorganisms, below 40°F will reduce metabolism. Many of the microbes involved in herbicide breakdown require oxygen, therefore increasing aeration can promote breakdown. Although water is required for a substrate, if a field remains underwater for a good portion of the year, an anaerobic (lacking oxygen) condition may occur, resulting in decreased breakdown and possibly carryover. However, this is not the case with the dinitroanalines (trifluralin, pendimethalin) which are readily broke down in anaerobic conditions. pH Soil pH can either decrease or increase a herbicides activity. Soil pH influences herbicide activity by having an effect on all of the processes mentioned above. Often it is associated with carry over problems. The pH value refers to the concentration of H+ ions in solution. This is an inverse relation, so if the soil is acidic (low pH) there is a high concentration of H+ in the soil solution and if it is basic (high pH) then the concentration of H+ is low. Optimum pH values for crop production generally lie between 5 and 7. The soil pH affects the amount of ionized herbicide molecule in solution. The pH will also affect the amount of charge that will occur on the soil particles. This in turn will have an effect on the amount of a herbicide that will adsorb to soil particles. If less herbicide is adsorbed to soil then more of the herbicide is available to leach. Soil pH has a strong influence on many of the chemical reactions that degrade herbicides in the soil. In the case of sulfonylureas, a high pH results in decreased hydrolysis leading to a possible carry over situation. As may be expected, pH also influences the growth and activity of living organisms. Many of the soil microbes have optimum growth between pH 6.5 to 8. Table 2 lists some of the herbicides that are affected by soil pH and the resulting effect the pH has on it. For more information on Herbicide and Soils interactions, the following books are a good source. R. J. Hance, ed. Interactions Between Herbicides and the Soil. Academic Press. New York: 1980. Thomas J. Monaco, Stephen C. Weller, and Floyd M. Ashton. Weed Science Principles and Practices. Fourth ed. John Wiley & Sons, Inc. New York: 2002 Wood Powell Anderson. Weed Science Principles and Applications. Third ed. West Publishing Company. St. Paul: 1996.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||