Pest & Crop Newsletter
|
|||||||||
Soybean Aphid in Indiana- (John Obermeyer, Rich Edwards, and Larry Bledsoe)
Soybean aphid, Aphis glycines Matsumura, was found on June 18 at the Agronomy Research Center, Tippecanoe County, on V3 soybean plants. This indicates that soybean aphids are now moving from their winter host, buckthorn, onto their summer one, soybean. This is our first observation, not an alert of an economic infestation. States in the northern Corn Belt observed this movement about one week earlier than we did. Groups attending Diagnostic Training Center sessions at the Agronomy Research Center looked for aphids as part of their training activity, but only ONE was found among such critters as thrips, spider mites, and whiteflies.
Soybean aphid has a very complicated lifecycle. Simply put, female aphids feed-on and reproduce in the summer on soybean. Females give birth to female offspring, so aphid numbers can increase quickly on soybean (it is estimated that populations can double every 2-1/2 days). In the fall, as temperatures drop and days grow shorter, a generation of winged females and males are produced. Both migrate from soybean to their overwintering host plant Rhamnus, a shrubby tree also known as buckthorn. Eggs are laid on buckthorn, which overwinter and hatch in the spring. Aphids emerging in the spring are females. After several generations on the overwintering host, winged spring migrants fly to soybean to establish new colonies. The soybean aphid feeds by using a needle-like, sucking mouthpart to remove plant sap. Plant damage occurs from large numbers of aphids removing a significant amount of water and nutrients as they feed on leaves and stems. Some isolated fields in east central Indiana, in 2001 had plants that were covered with aphids, and leaves that were curled and wilted. Leaves on the bottom-third of plants were covered with shed aphid skins (resembling white powder) and aphid secreted honeydew, both of which are signs of aphid presence. Gray sooty mold growing on the honeydew, also covered these leaves. Plants covered with aphids were often stunted when compared to plants from other parts of the field. In some cases, heavily infested plants showed dramatic leaf yellowing. This yellowing may have been associated with potassium (K) deficiency, because symptoms can be more pronounced in fields where both high numbers of aphids and deficient levels of K are found. It is too early to speculate on how severe the infestations will be in the Midwest, much less Indiana, for this season. Considerable time and effort has been and will be devoted to this pest throughout the Corn Belt because of its potential economic impact on soybeans. Indiana has had minimal crop damage due to this aphid since its discovery in 2000. Therefore, our expertise in this area relies heavily on what we read and hear from colleagues in neighboring states. Many pest managers are asking about thresholds for this insect in case outbreaks occur. Christina DiFonzo, Michigan State University Entomologist, put together a treatment decision “checklist” this past winter. Her checklist follows: Aphid distribution: Aphids on leaves and stems. When aphids begin to move from the undersides of leaves onto stems, the population is large and increasing. Aphids on stems generally are easy to see without a hand lens. Aphid number: Leaflet rating of at least 3.0. The leaflet rating is fairly quick and easy to do, and will allow you to assess aphid numbers after treatment. A rating of a 3.0 is a minimum of 25 aphids on every leaflet of the plant. Plant appearance: Honeydew (sticky substance) on plants. Honeydew is a sugary substance secreted by aphids as they feed. It is mainly an annoyance, although it promotes the growth of gray sooty mold on leaf surfaces. Honeydew is a sign aphid numbers are large. Aphid appearance: Healthy. Aphid-infesting fungi already exist in your fields, in the soil and on plant surfaces. These fungi specifically attack and infect aphids and can crash the aphid population in a field in a matter of days. Infected aphids are pinkish, white, or tan, and fuzzy from the growth of fungi out of their bodies. When weather conditions are favorable, the fungi can infest and control aphids quickly. Once a fungal infection starts, an insecticide spray may not be needed. Weather conditions: Warm and dry. Aphid pathogenic fungi reduce aphid numbers best in warm, humid weather. Under dry conditions, these fungi cannot infect aphids. When thinking about aphids and weather, think about the same conditions favorable for spider mite infestation in soybean. Timing: July. June is likely too early to assess aphid populations and make a spray decision. August is probably too late to get the most yield advantage from treatment. Plant stage: Flowering and early pod development. Flowering and early pod fill seem to be critical times for aphid control. Large numbers of aphid feeding on the plant may cause flowers and pods to abort. Also, there is Minnesota data showing that node number was reduced by large numbers of aphids. Spraying too late in the season, once pods are formed, is probably too late to get the most yield advantage from treatment.” As well, predatory insects, especially lady beetle adults and larvae, lacewing larvae, and syrphid fly larvae, have been very abundant in infested fields and should provide some control, if present. Parasitic wasps, which lay eggs directly into aphids, have been less abundant, but still present. In addition to the above-mentioned pathogenic fungi, these biocontrol agents have the potential to dramatically reduce aphid numbers in Indiana to below economic levels. Efficacy trials conducted by Michigan State and Minnesota demonstrated that many products control aphids in soybean. Complete coverage on the foliage, as with spider mites, seems to be the key. Last year, on-farm trials conducted in Michigan yielded from 2 to 24 bushels better than the untreated. Yield benefits decreased the later applications were applied in the season. Further information with many color pictures can be found in extension publication E-217, Soybean Aphid (new May 2001). A hard copy of this publication can be obtained by calling 765-494-8491 or an electronic copy viewed at <http://www.entm.purdue.edu/entomology/ext/targets/e-series/e-list.htm>.
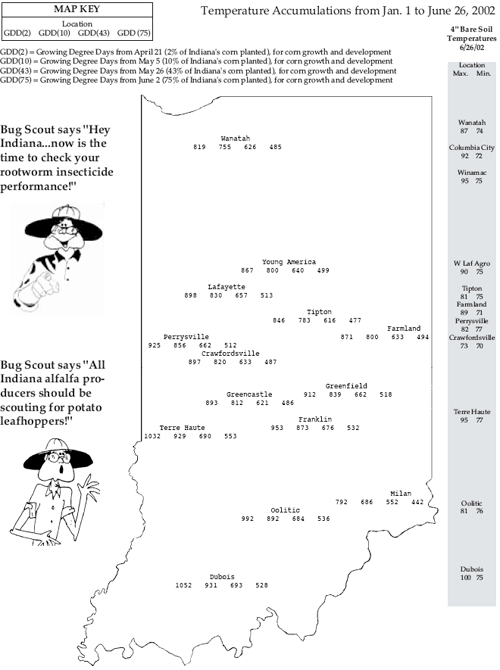
Rootworm Damage Being Reported on Late-Planted Corn– (John Obermeyer, Larry Bledsoe, and Rich Edwards)
This past week, pest managers were out diagnosing problem fields. Symptoms that have been commonly observed in several central Indiana cornfields are plants lagging behind in growth and showing yellowish color. Purdue specialists, Bob Nielsen and Greg Shaner, have visited some of these fields, as have several company agronomists. Much of this corn was planted in late May/early June without the application of an insecticide. Inspections of root systems have revealed tunneling and pruning by rootworms. In addition, poor nodal root development from dry soil surface and compacted soils from poor planting conditions have compounded damage. Rootworm larval feeding causes reduced water and nutrient uptake and can lead to standability problems. Major yield losses occur when nodal and brace roots are so severely damaged that plants lodge. Research has shown that even though plants attempt to right themselves (goose neck or sled-runner look), on average, there is at least a 30% yield loss. Obviously, losses will be more dramatic if winds blow the plants over shortly before or at pollination. By the time you read this, rootworm development will be too far advanced, except in the most northerly counties of Indiana, to attempt rescue treatments with insecticides. If still possible, cultivation may help root regeneration by throwing soil up at the base of damaged plants. The best defense you have against rootworm is scouting soybean fields for beetles this August and determining the potential for egg laying and subsequent damage to next year’s corn. This suggestion is for all of Indiana. Further information with many color pictures can be found in extension publication E-218, Monitoring and Decision Rules for Western Corn Rootworm Beetles in Soybean (New 5/2001). A hard copy of this publication can be obtained by calling 765-494-8491 or an electronic copy viewed at <http://www.entm.purdue.edu/entomology/ext/targets/
Potato Leafhopper Populations on the Rise– (John Obermeyer, Rich Edwards, and Larry Bledsoe)
Populations of potato leafhopper in alfalfa fields and black light traps have been rising throughout the state. Several observations of high numbers of leafhoppers coming to lights at night have been shared with us. Undoubtedly, the warmer temperatures have contributed to this increase. Producers are encouraged to inspect new growth soon after cutting for potato leafhopper; this is when alfalfa is most susceptible to feeding, leading to reduced yields and protein levels. Remember, once yellowing or “hopper burn” is seen, the damage has already been done. Refer to Pest&Crop #13, for sampling and management guidelines. For recommended insecticides, see E-220, Alfalfa Insect Control Recommendations – 2002. This and other field crop related publications can be viewed electronically at <http://www.entm.purdue.edu/
Stalk Borer Interfering With Herbicide Uptake– (John Obermeyer, Rich Edwards, and Larry Bledsoe)
Darrel Daniels, BASF, has made us aware of some poor performance complaints with post-emergence herbicide applications on giant ragweed. During field surveys, it was noted that some plants were wilting due to factors not related to herbicide activity. Some ragweed plants were split open and revealed extensive tunneling by stalk borer larvae. Obviously, this insect’s tunneling activity prevented sufficient translocation of the herbicide in the ragweed plants to kill them. Not only is giant ragweed a favorite egg-laying site for stalk borer moths in the fall, but also a host for the larvae in the spring. Stalk borer can be an efficient biocontrol agent for ragweed, however, it can also kill adjacent plants, such as corn, thus limiting its usefulness as a weed control agent.
|
|||||||||
Lorsban 15G and Callisto– (Glenn Nice and Thomas Bauman) We have received questions about the use of Lorsban 15G (Dow AgroSciences) and Callisto (Syngenta). Dow AgroSciences has a 2(ee) recommendation for the use of soil applied Lorsban 15G insecticide as a T-band application at 8 oz/A before a POST application of Callisto. These recommendations have been based on field trial and greenhouse data collected by Dow AgroSciences, indicating that injury should not occur. Syngenta’s label for Callisto states that injury may occur when Callisto is applied POST where Lorsban has been used. It is NOT recommended by either company that Callisto be applied PRE when Lorsban 15G has been used or that Callisto be tank mixed with any organophosphate or carbamate insecticide. If you find that you have suspect injury after using Lorsban as prescribed above and Callisto POST contact your Dow AgroScience representative.
|
|||||||||
Too Much Dang Stress- Recap– (Glenn Nice and Thomas Bauman)
Several weeks ago, I offered my two cents’ worth on some of the causes of the yellow, stunted corn in early-planted fields throughout the northern third of Indiana this spring (P&C Newsletter, 14 June). With this article, I want to reinforce the notion that a number of stresses are to blame this year and that every situation likely results from a different set or combination of stresses. That is the reason why the recuperation of these plants has been so variable from one field to the next. Stunted plants in some fields have recovered nicely, while in other fields the plant appearance has scarcely improved, while in other fields the plants eventually died. Rescue applications of various fertilizers (including ammonium sulfate, magnesium sulfate, manganese sulfate, 28% UAN, anhydrous ammonia) have either improved plant appearance dramatically, some, or not at all compared to untreated areas of the field. Field location is one of the first clues in determining the likely causes of the stunting. In some fields, the plant stunting occurred in the lower, poorly drained areas and was accompanied by significant stand loss. Seed rot and/or seedling blight were the most frequent causes of the problem in these fields, encouraged by the slow initial pace of corn development that delayed the successful establishment of the nodal root system. In other fields, the stunted corn was most frequently located in the sandiest, seemingly better drained, areas of the fields. Plant stunting and yellowing were visually dramatic, but not what you call severe. With the warm-up that began in late May, many of these fields have recovered to some extent on their own. Causes of the stress in these fields was some combination of cool weather, lower than desired soil pH, minor seedling blight, and leaching of mobile soil nutrients such as nitrogen and sulfur below where the young corn plants’ root systems could yet explore. Other, more severe, cases of stunted, yellow corn in sandy soils have not recovered well. In addition to the severely stunted above-ground plant parts, the root systems of these plants are also severely stunted and/or malformed in their development. In some cases, the root symptom was similar to that which we used to attribute to trifluralin (Treflan) carryover when that herbicide was commonly used in soybean. Interestingly, seedling blight was also evident on the mesocotyl and seed roots of many stunted plants in the sandy areas as well as an interesting yellow discoloration of the roots. Potential causes of the severe stunting in these sandy areas include leaching from frequent heavy rains of nitrogen and/or sulfur below the extent of the young plants’ root systems, compaction of seed bed or furrow sidewalls by tilling or planting “on the wet side” (yes, sand will compact!), sandblasting injury to young plants during windy days, extremely low soil pH, and corn nematodes. The latter cause is one that I frankly rarely consider when troubleshooting stunted corn, but I have learned this spring that it can be a significant stress to young corn plants under the right (or wrong) conditions. I submitted a plant and soil sample taken from a severely stunted area of corn on a sandy knoll in northwest Indiana to Purdue’s Nematology Laboratory late last week which was subsequently diagnosed as being severely infested with needle nematodes. Injury from corn nematodes is not unheard of in sandy soils of northern Indiana, but is particularly encouraged by lengthy periods of wet and cool soils when young corn (emergence to V3) is developing very slowly. Additional stress to the plants by low soil pH (low 5’s or less) and nutrient deficiencies (albeit even if temporary) further limits the young plants’ abilities to “outgrow” the damage caused by the nematodes. The additional stress caused by the recent hot and dry conditions further stressed the already limited root systems and limited the plants’ abilities to recover. Jamal Faghihi forewarned us back in early May that conditions were ripe for nematode damage to corn (P&C Newsletter, 3 May). Information about needle nematodes can be found in Purdue Extension publication E-215 available at your local county Extension office or on the Web at <http://www.entm.purdue.edu/Entomology/ext/
Forage Testing and Balancing Rations Essential to Cost Effective Livestock Farms– (Keith Johnson)
Testing forages for nutritional quality is an important component of a cost-effective livestock enterprise. Cash crop hay producers can use test results as an effective way to market excellent quality hay and to appropriately price hay for sale. My interactions with forage/livestock producers suggest that more forage testing should be done than what actually does occur. Dairy producers probably do the best job of measuring forage quality and following through with balancing rations than any other livestock group; by milking their livestock two or three times a day, they can quickly see the impact that forage quality can make in the performance of their dairy animals. It is more difficult for meat animal producers to see performance changes as affected by forage type as they don’t have the daily production measure. Much of the first cutting hay was tardy this year because of persistent rain. As a result, forage quality will be lower because the crop was overly mature for optimum quality. When a crop is overly mature, protein content will be lower and fiber content will be higher, which reduces consumption and digestibility. The uninformed producer that feeds overly mature first-cutting hay as though it was harvested in timely fashion will be disappointed with the performance of their livestock. I would suggest that all forage harvests be inventoried by harvest and field location (lot), and placed in storage so that sampling of several lots can be done with least hassle. A hay-sampling probe, as compared to a hand sample or use of scissors, is the best way to collect a representative sample. Each lot of hay should have approximately 20 probes taken to best represent the forage quality of the hay. One probe from each of 20-small rectangular bales or two probes from each of ten-large round or large rectangular bales should comprise the sample. Each sample (the 20 probes) should be placed in a plastic bag and sealed; zip lock bags work well. The sample should be properly identified with information specific to that lot of hay. There are some excellent forage testing laboratories in the region. You may want to ask your feed company representative about the laboratory that they use for feed analyses, or confer with a Purdue Cooperative Extension Service Educator about laboratories in your area. Sample submission forms should be acquired from the laboratory that does the analytical work. The completed form should accompany the samples to the laboratory. After results are obtained, share the information with an individual that has been trained to balance rations and follow through with needed supplementation to best assure that livestock performance is not reduced.
|
|||||||||
Forage Management Training Session on September 5-(Keith Johnson) If forage management skills need to be improved, then the September 5 training session at the Purdue University Diagnostic Training and Research Center is for you. The Center is located at the Agronomy Research Center which is located northwest of West Lafayette. Details will be forthcoming. Preregistration will be required. Certification credits will be requested. Come join us at this hands-on training session. I guarantee that participants will leave the training session with grass stains on their knees, dirt under their fingernails, the aroma of silage on their hands and clothes, and some knowledge to use, too. Visit the Purdue Forage Information web site after mid-July for details. <http://www.agry.purdue.edu/ext/forages/>.
|
|||||||||
|





 Hopper burn on alfalfa
Hopper burn on alfalfa Stalk borer and damage in giant ragweed stem.
Stalk borer and damage in giant ragweed stem.






