Pest & Crop Newsletter
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Mild Winter Temperatures and Field Crop Insects– (John Obermeyer, Rich Edwards, and Larry Bledsoe)
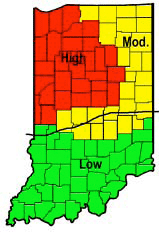
Even the most casual weather observer can’t ignore the fact that the weather this fall and winter, at least up to this week, has been mild. Data on ambient and soil temperatures back this up. Will this equate to large insect populations and serious crop damage this coming season? As you probably already guessed...it depends! Insect predictions are as reliable as NCAA basketball tournament picks. Although we can’t tell you for sure what will happen with these critters coming out of this winter, we can give you some information on insect/environment/crop interactions that might clear the picture some: Above Ground Insects: Bean Leaf Beetle Corn Flea Beetle European Corn Borer Black Cutworm Alfalfa Weevil Below Ground Insects: Western Corn Rootworm White Grubs Perceived Corn Rootworm Risk Area, 2002
Rootworm Insecticide Classifications and Consistency of Performance - (John Obermeyer, Rich Edwards, and Larry Bledsoe)
Click for Table.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Virus Diseases of Wheat- (Gregory Shaner)
As wheat begins to grow, we may see widespread yellowing that looks like nutrient deficiency. It is possible that these symptoms will be the result of infection by either to of two soilborne viruses of wheat are widespread in Indiana soils, rather than to a deficiency of nitrogen. These viruses are Wheat spindle streak mosaic virus (WSSMV) and Soilborne wheat mosaic virus (SBWMV). Both viruses persist in a common soilborne fungus, Polymyxa graminis. Spores of this fungus infect wheat roots and in so doing transmit the virus to the wheat plant. Cool, wet soils favor infection. Much of the state experienced those conditions last fall, so there is a good chance that these diseases will appear this spring. Weather conditions in the spring are also critical for symptom expression. These diseases are often most conspicuous when a period of unusually warm weather early in the spring is followed by a return to cooler weather. At that time, symptoms can suddenly appear in many wheat fields. The symptom to look for is a pale green to yellow mosaic on young leaves. SBWMV, as its name implies, causes a mosaic – narrow, pale green to yellow, wavy-margined streaks on the leaf blade. Symptoms of WSSMV infection are similar, but the streaks tend to taper at both ends, hence the name “spindle.” From a distance, fields or parts of fields have a pale green or yellow appearance, as though they are deficient in nitrogen. In practice, it is very difficult to distinguish these two diseases based on symptoms. Both viruses may be found in the same field, and both viruses may infect the same plant. Wheat spindle streak is reported to be more uniformly distributed throughout fields than is soilborne wheat mosaic virus. Most varieties of soft red winter wheat grown in Indiana have some degree of resistance to these viruses. They may show some yellowing during periods of fluctuating temperatures during the spring, but once the cold weather is past, these varieties tend to outgrow the symptoms on lower leaves and there is probably little damage. A few varieties are more susceptible. The intensity of yellowing is greater, and is accompanied by stunting, reduced tillering, and death of some plants in the field. These varieties will suffer economic damage from these diseases. There is no remedial action that can be taken at this stage. If a variety develops severe symptoms, don’t plant it again next year. There are plenty of varieties with good resistance.
Risk of Yellow Dwarf in Wheat – (Gregory Shaner)
Yellow dwarf is caused by either of two closely related viruses: Barley Yellow Dwarf Virus (BYDV) and Cereal Yellow Dwarf Virus (CYDV). Until recently, these two viruses were considered to be one species (BYDV), consisting of different serotypes. Two of the serotypes have now been transferred to a “new” virus, CYDV. These viruses can only be transmitted to wheat by certain species of aphids. If an aphid feeds on an infected plant, it acquires the virus, and then can transmit it to another plant when it feeds there. Infection can occur in the fall or in the spring. Fall infections are more damaging, although they may not result in conspicuous leaf discoloration symptoms. However, plants infected in the fall will be stunted, won’t tiller well, and may be more subject to heaving and winterkilling than uninfected plants. Leaves tend to be upright and less flexible than healthy leaves. On cultivars that do show yellowing leaves, the symptoms may look like a nutrient deficiency. BYDV and CYDV survive in living host plants (cereals or various grasses). Aphids that feed on infected grasses or volunteer cereals in the fall and then move into the newly emerging wheat crop can transmit the viruses. The extent of infection by these viruses depends on aphid activity and population sizes.. Once infection is established in a field, more plants can become infected as aphids move from plant to plant. I saw symptoms that looked like yellow dwarf last November in some early-planted wheat. One of the reasons for observing the fly-free date for wheat sowing is to reduce the likelihood of infection by BYDV or CYDV. The cooler temperatures that normally occur after the fly-free date mean than aphids are less active. However, the long, warm fall of 2001 provided opportunity for aphids to remain active longer than normal and move into wheat that was sown even after the fly-free date. So, this spring, we may see yellow dwarf on wheat sown over a wide range of dates. There are no remedial steps that can be taken at this stage against yellow dwarf. Cultivars differ in susceptibility. Most cultivars have some resistance. If yellow dwarf is suspected, the only reliable means of diagnosis is a serological test that detects the viruses in plant sap. Suspect samples can be sent to the Purdue Plant Diagnostic Lab or diagnostic labs in other states for confirmation of yellow dwarf.
Growth Stages of Wheat– (Gregory Shaner)
For effective management of wheat, it is important to recognize the stages of growth as the crop develops. Heading date is a commonly-recognized indicator of relative maturity, but the ability to recognize other growth stages is important for judging the progress of the crop and making management decisions, such as application of fertilizer, herbicides, or fungicides, and for predicting the consequences of disease or injury to the crop. In the Feekes scale, growth stages are divided into 11 major categories. Head emergence, flowering, and grain filling (Feekes Growth Stages 10 and 11) are further subdivided. The Feekes Growth Stage scale is presented below, with a description of crop development stage that corresponds to each number. Corresponding Decimal Growth Stages are given in brackets.
There are 9 major divisions (1-9) in the Decimal scale, with 10 possible subdivisions (0-9) for each major division. For example, the tillering stage is denoted by 2 in the Decimal scale, and the second digit indicates the number of tillers per plant. The Feekes scale simply notes whether tillers have begun forming (FGS 2), or whether tillering is essentially completed (FGS 3), without requiring the counting of tillers, although tiller number per plant could be appended after the “2”, e.g. FGS 2.4. In winter wheat, the period from beginning of tillering to completion of tillering may extend for a considerable time, from autumn into the following spring. Likewise, the precise limits of FGS 4 and 5 are not clear. Depending on planting date, variety, and weather in the fall, plants may reach the pseudostem erection stage in the fall, or only in the spring as the crop comes out of dormancy. Jointing (FGS 6) can be clearly determined. The original Feekes scale simply defined stage 6 as when the first node was visible at the base of the shoot. The Decimal scale provides a more precise definition for this stage, namely when the distance between the crown and the first stem node is at least 1 cm (0.4 in.), and I have included this in the growth stage table. When the second aboveground node is at least 2 cm (0.8 in.) above the first node, the plant has reached FGS 7. The stage when the flag leaf first appears (FGS 8) is important for application of a foliar fungicide. Soft red winter wheats in Indiana typically have 4 aboveground nodes when fully developed. The sheath of the uppermost leaf (flag leaf, F) arises from the top node. The leaf below the flag leaf (F-1) arises from the next node down, etc. Thus, leaf F-3 arises from the lowest aboveground node. The lowest aboveground node is near the ground when it first appears, but will move upward somewhat as the stem elongates. At FGS 8, there are usually two clearly differentiated nodes on the stem. The lowest will average about 7 cm (2-3/4 in.) above the soil line. The second node (from which leaf F-2 arises) will be about 15 cm (6 in.) above ground. The third node will usually be visible, but because it is only about 1 cm (0.4 in.) above node 2, it is not counted. As the wheat continues to grow, the distance between nodes increases, and the fourth node becomes evident. Flowering in wheat begins roughly in the middle of the head and progresses both upward and downward. Flowering at a given position in the head can be judged by the presence of extruded anthers. Ripening is judged by removing developing kernels from the center of several heads and determining whether the contents are watery, milky, or at the soft or hard dough stages. By the time wheat has reached FGS 8, leaves F-5 and below are usually withered, from infection by Septoria, Stagonospora, and other fungi. The next leaf up (F-4) usually withers about the time heads have fully emerged. In the absence of Septoria and Stagonospora blotches, powdery mildew, or other foliar diseases, leaves F-3 through F should remain green until the wheat approaches maturity. Often, however, disease destroys leaves at each layer of the canopy prematurely. Fungicide control is aimed at maintaining these leaves, particularly F and F-1, in a healthy condition. If a grower is planning to apply a fungicide at flag leaf emergence (FGS 8), it would be helpful to know when that stage will be reached, relative to some earlier, easily determined growth stage. The jointing (FGS 6) and 2-node (FGS 7) stages can be determined with precision if a wheat field is monitored frequently. The time required for a plant to progress from either of these stages to FGS 8 is not constant. It depends on weather conditions, particularly temperature. Over many years, we have monitored wheat crop development in various field trials, and the following observations can give some guidelines for the average and ranges of times required for plants to progress from one growth stage to another. We found that it takes about a week to progress from FGS 6 to FGS 7, and another 8 days to go from FGS 7 to FGS 8 (with a range of 5 to 10 days). It can take from 3 to 8 days for the flag leaf blade to fully expand (going from FGS 8 to FGS 9). It can take from 9 to 16 days to progress from FGS 9 to full head emergence (FGS 10.5) or the beginning of flowering (FGS 10.5.1).
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Wheat Update– (Charles Mansfield and Ellsworth Christmas, Agronomy Dept.) Wheat in southern Indiana is already out of dormancy and beginning to grow. There is some concern about susceptibility to cold damage and survivability of wheat should cold weather occur. Wheat that is completely dormant can withstand temperatures down to approximately -10 oF. Once out of dormancy some winter hardiness is lost and temperatures in the low teens can cause injury. See Table 1 for specific growth stages and critical temperatures that could cause injury. Plant response is based on exposure of wheat to these temperatures for at least two hours.
From the table one can see that if there is an average of less than 15 plants per square foot, that field is not a likely candidate for good yield. Weed pressure would also be a real concern in thin stands. On the other hand, if there are 25+ plants per square foot, the outlook is much better for high yield potential. Between 15 and 25 plants per square foot the potential outcome becomes less clear, and other factors like weed species, weed pressure, alternate use of the land, etc.. play more important roles in the decision making process.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Bug Scout
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||