Pest & Crop Newsletter
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Winter Temperatures, Corn Flea Beetle Survival, and Potential for Stewart's Wilt– (John Obermeyer, Rich Edwards, and Greg Shaner)
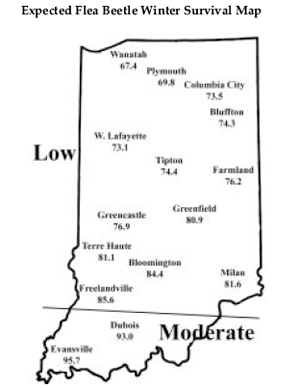
Winter temperatures have a direct impact on how well the corn flea beetle overwinters. This is especially important since this insect can vector and transmit the bacterial disease, Stewart’s wilt. The severity of the disease correlates well with winter temperatures, because the bacteria survives in the gut of the overwintering beetle. Warmer temperatures result in higher beetle survival, and greater potential for disease problems to develop. To determine the potential severity of Stewart’s disease, add the average daily temperatures for the months of December, January, and February. If the sum is below 90, the potential for disease problems to develop is low. If the sum is between 90 and 100, moderate disease activity is a possibility. Sums above 100 indicate that there is a high probability that severe problems will develop for susceptible corn. To help you better gauge the potential for corn flea beetle activity in your area, and thus the potential severity of the threat of the disease, we have created the following state map. Thus, according to the temperature model there is a low probability of corn flea beetle activity and subsequent disease throughout most of Indiana; areas south of approximately US 50 have a moderate chance of beetle and disease activity. This temperature model for corn flea beetle has been around many years and has been fairly accurate in predicting the activity of this pest the following spring. However one inherent flaw is that the model is based on ambient air temperatures, not temperatures under leaf litter and grass clumps where this pest overwinters. As well, snow cover, which can provide an excellent insulating blanket for the insect, may protect some beetles from winterkill. Even with this “disclaimer” statement, we think the 2000/2001 winter was cold enough to have negatively impacted overwintering beetles. There are two phases of this disease: a wilt phase and a leaf blight phase. In the wilt phase, plants wilt rapidly, usually at an early stage of growth. Sweet corn hybrids are especially susceptible. Some dent corn inbreds, and occasional dent corn hybrids, and some popcorn lines are susceptible as well. Dent corn hybrids rarely wilt after growth stage V5. Leaves emerging from the whorl of infected plants are often the first to wilt. Internal tissues at the growing point are discolored or hollowed out. Faint green to yellow streaks containing corn flea beetle feeding marks are visible on one or more leaves. If stalks of wilted plants are cut, it may be possible to see yellow, moist beads of bacterial ooze. The leaf blight phase can occur at any time during the growing season, but often does not appear until after tasseling. Lesions are long and narrow, with pale green to yellow streaks and irregular or wavy-margins. Streaked areas die and become straw-colored. Severely infected leaves may die prematurely. Management decisions made now, should be based on the corn’s susceptibility to the disease and the number of beetles anticipated. Low susceptibility - pest managers should scout fields and apply a foliar rescue treatment if damage is severe, there are 5 or more beetles per plant, and the seedling is growing slowly (e.g., cool temperatures). High susceptibility - sample field edges (i.e., overwintering sites) before or immediately following planting to assess the potential beetle movement into the field. A sweep net is an ideal sampling tool for this pest. If any beetles are discovered, an insecticide application is warranted. Three systemic soil insecticides that should give good control of flea beetle are available at planting, Counter CR, Furadan 4F, and Prescribe treated seed. Counter may cause inbred damage where post-grass sulfonylurea herbicides are used. Furadan may require re-tooling the planter for liquid application. Prescribe (and Gaucho Extra for inbred seed) must be applied to seed by commercial seed treaters. Prescribe is labeled for fleas beetle control through the 5th leaf stage. If a systemic soil insecticide is not an option, broadcasted foliar insecticides at the time when corn spikes should provide 7 to 10 days of residual protection from beetle feeding. CAUTION: treating of field edges and waterways for beetle control may be an off label application. Avoid movement of insecticides, including soil-bound materials into aquatic environments.
Some of the Newer Rootworm Larval Control Products– (John Obermeyer, Rich Edwards, and Larry Bledsoe)
In Pest&Crop #1, February 26, 2001, we included a table within the article “Criteria for Treating First-Year Corn for Rootworm, 2001.” This table, “Factors to Consider When Choosing a Corn Rootworm Soil Insecticide,” shows the consistency of performance of six products that have been tested for at least 3 years. We continue to receive questions concerning the rootworm efficacy of the newer chemistries and/or application technologies that are now available. Below is a summary of these products and ONE year’s efficacy data from several states. Capture 2EC (bifenthrin) is a liquid insecticide with a new registration for rootworm larvae control. Previously, this synthetic pyrethroid insecticide was only used as a foliar spray on corn for above ground insect pests (e.g., cutworm, corn borer, etc.). Capture for rootworms must be applied at 0.3 fluid ounces per 1,000 foot of row in a 5-7 inch T-band over an open seed furrow in front of the press wheel. As the label states, “apply in a minimum of 3 gallons of finished spray per acre.” Obviously, the planter will need to be rigged for liquid application, and it can be mixed and applied with pop-up fertilizer. Prescribe (imidacloprid) is one of the new coated seed insecticides. This chloronicotinyl chemistry is new to field crops. It is expected that more, and presumably improved, compounds of this insecticide class are coming in the future. Prescribe is applied to the seed at a very precise rate of 1.34 mg/kernel only by commercial seed treaters. Producers must purchase their seed pre-treated from participating seed companies. Note of caution, the label states “suppression” for corn rootworm and it is likely that severe rootworm infestations will not be controlled. ProShield (tefluthrin) is another coated seed with the same active ingredient as in Force 3G granular insecticide, a synthetic pyrethroid). ProShield is a patented process of micro-encapsulation and polymers that is only available on Syngenta hybrids. There was a limited supply of ProShield last year for producer testing. Flowability of the seeds within the planter boxes was noted as a problem last year and company representatives tell us that the “bugs” have been worked out for 2001. Below are efficacy trials from Indiana, Illinois and Iowa for 2000:
Potential for Problems with Bean Leaf Beetle Highly Variable– (C. Richard Edwards, John Obermeyer, and Larry Bledsoe)
Bean leaf beetle (BLB) numbers were at very high levels last spring in several areas of Indiana. Some soybean fields were rescued with foliar insecticides because early planted, slow growing plants were riddled by hungry beetles. By mid-summer, there was discussion that these beetles not only caused defoliation damage, but also vectored the disease bean pod mottle virus (BPMV). An excellent introductory discussion concerning this disease is available in Iowa State University’s April 24, 2000 newsletter Integrated Pest Management which can be seen on the web at <http://www.ipm.iastate.edu/ipm/icm/2000/4-24-2000/newsoyvir.html>. Recently, we received a report that at least one seed company is recommending that soybean producers add a foliar insecticide for BLB control to their post emergence herbicide(s) in 2001 (especially Roundup®, but could be others as well). It appears that this recommendation is being given to producers to reduce the chance of BLB feeding and BPMV showing up in commercial soybeans. We have some major problems with this approach. First, it is unlikely that BLB numbers will be as high this spring as over the past two to three years. BLB adults overwinter utilizing leaf litter and other ground cover to shield themselves from old man winter (refer to the corn flea beetle article in this issue of the Pest&Crop, this may be a good indicator on how well the BLB overwintered). Therefore, we expect lower populations of BLB and subsequent damage this spring. Although the transmission of this virus by BLBs is possible, there is no guarantee that the virus will be present. Even if the beetles are present in high numbers and the BPMV is present, applying an insecticide at the time of herbicide application may not be the proper timing for the spray (if BLBs are present before the application and are vectoring the virus, then the virus will have already been transmitted). Since the herbicide will be sprayed at a time that is most conducive for controlling weeds and not for BLB management, the chance of “catching” the BLBs at the proper time to reduce the risk of BPMV transmission is an expensive “hit or miss” situation at best. The timing of BLB emergence and its movement to soybeans is highly variable. Yes, BLBs can vector BPMV and cause yield losses, but it is not a given. Transmission of BPMV to soybeans can take place through the beetle picking up the virus while feeding on wild or cultivated legumes. However, using the application of herbicides as a convenient way to apply the insecticide is not a “best” management practice. What producers should do is scout their soybeans for BLB and follow established management guidelines. Perhaps what producers should do is check with their seed supplier to find out how susceptible the seed companies varieties are to BPMV. If the virus is of major concern, the producer should grow a soybean variety that is more tolerant to BPMV. We are very concerned about the economical and environmental consequences of applying insecticides as routine applications with herbicides when their need is not based on scouting information and good pest management information. For more information on the BLB see the Field Crops Pest Management Manual (IPM-1) or Extension Publication E-51, Bean Leaf Beetle on Soybeans. These publications are available from the Media Distribution Center by calling 1-888-398-4636. E-51 can also be found on the Extension Entomology Web page <http://www.entm.purdue.edu/entomology/ext/targets/publicat.htm>.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Herbicide Resistant Weed Problems Increasing in Indiana: Add Shattercane to the List- (Case Medlin and Tom T. Bauman)
In recent years, herbicide resistant weeds have become an important issue to consider when making weed management decisions. Herbicide resistant weeds can, and have, developed from natural weed populations. We have known for a number of years that populations of triazine (the herbicide family of Atrazine and Princep) resistant pigweed (Amaranthus spp.), lambsquarters (Chenopodium album), and jimsonweed (Datura stramonium) have been in the state. These populations have generally been found in areas where corn has been grown for consecutive years. Likewise, surrounding states have reported weed resistance in field crops following the continuous use of certain herbicides. Within the last few years, we have documented giant ragweed (Ambrosia trifida) populations that are resistant to an important ALS inhibiting herbicide, FirstRate. In most cases this resistance has occurred in fields with histories of continuous use of ALS inhibiting soybean products (primarily Pursuit and Scepter). This is a prime example of cross-resistance occurring in a species to products in the same herbicide family, but with slightly different active sites. Just this winter, we documented a shattercane (Sorghum bicolor) population in southern Indiana that is resistant to at least one ALS inhibitor, Accent. Susceptible and resistant shattercane plants were used in our study. Plants were sprayed with 1, 2, 4, or 6 oz/A of Accent. See the photos for results.
Herbicide Mode-of-Action Categories– (Merrill A. Ross and Case R. Medlin)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fungicide Seed Treatment for Soybeans– (Gregory Shaner)
Ellsworth Christmas reported in the first issue of Pest&Crop #1, February 26, 2001, that the quality of soybean seed produced in Indiana is good. Seed produced in Iowa and Nebraska is not of good quality this year. Because seed may be moved from one area to another, growers should pay careful attention to the seed they purchase this year. Although quality of soybean seed produced in Indiana last year is generally good, Phomopsis seed decay was a problem in some areas. This fungus is common in fields. Pods can be infected anytime after they are formed, but seeds do not become infected until after the plant has reached physiological maturity. Warm, wet weather during the interval between physiological maturity and harvest favors seed infection. Seed that is visibly infected (shriveled, cracked, and with a chalky appearance) should not be used for planting. However, seed that are normal in appearance may also be infected. A seed treatment fungicide can reduce the incidence of seedling blight associated with Phomopsis seed infection. Regardless of the presence of Phomopsis in seed, poor seed quality can increase the probability of seedling disease. Several common soilborne fungi can infect seed or young seedlings, resulting in stunting and premature plant death. Fungicide seed treatment can provide protection against these fungi. The need for seed treatment depends on weather and soil conditions as well as on seed quality. If seed is planted under less than ideal conditions or young plants are subjected to environmental stress, seedling blights are more likely to cause a problem. Seedling blight pathogens pose little threat to a soybean seedling that is growing in a warm, uncompacted soil with just the right amount of moisture. Conversely, planting seed into cold, wet soil increases the risk for seedling blights. Use of poor-quality seed increases the risk further. Because fungicide seed treatment is not a standard practice for soybeans, many seed dealers are reluctant to treat seed except on demand. There are several on-farm products, for application either as a hopper box treatment or for application to seed as it is augured into the planter. These include: Allegiance FL, Allegiance LS, Apron XL LS, Apron Max RTA, Prevail, Protector L/Allegiance, Stiletto, and System. Growers should read and follow label directions for both the applicator system and seed treatment material selected. Several of these fungicides contain an active ingredient (metalaxyl or mefenoxam) that is specifically targeted toward Pythium and Phytophthora, fungi commonly associated with seedling blight of soybean. Other active ingredients, such as thiram, captan, TBZ, fludioxonil, and carboxin, are active against other types of fungi, such as Fusarium and Rhizoctonia. It is probably best to use a seed treatment that contains a combination of these products in order to protect against both groups of fungi. Information on the relative efficacy of various seed treatment products can be found at the following Web site, published by plant pathologists at Ohio State University: <http://www.ag.ohio-state.edu/%7Ecorn/library/articles/soyseedtrt.html>.
Diplodia Ear and Stalk Rot of Corn– (Gregory Shaner)
Diplodia ear and stalk rot was widespread in Indiana last year. The fungus produces a gray mold that grows between and over kernels. Typically, the mold begins at the butt of the ear and progresses toward the tip. Infected kernels are light weight and shriveled. Dr. Charles Woloshuk, extension specialist for stored grain fungal problems, conducted a survey of random corn fields throughout Indiana and found Diplodia in ear samples from counties throughout Indiana. In some fields, many ears were infected. Diplodia ear rot was common in Indiana during the first half of the 20th century. From about the late 1960s through the mid 1990s, however, the disease all but disappeared. It has been seen several times since 1995 in isolated areas of the state, but last year it was widespread. The fungus that causes Diplodia ear and stalk rot (formerly Diplodia maydis, now Stenocarpella maydis) survives in corn residue. It is therefore logical to conclude that conservation tillage practices that leave corn residue on the soil surface have contributed to the reappearance of this disease. The story is probably more complicated than that, however. A recent study on the effect of tillage on Diplodia ear rot comes from South Africa. Although South Africa is a long way from Indiana, I think the findings from that work are generally applicable to our situation. In a study by Flett and others, published in Plant Disease (July, 1998, vol. 82, pages 781-784), several tillage systems were compared. Over the course of 3 years, the various “primary” tillage systems were interrupted with a moldboard plowing at right angles to the direction of planting. As might be expected, deep ripping (20 in.) or V-blade plowing (4 in.) with the rows left the most residue on the surface. Disking left less residue, and plowing in the direction of the rows left even less. When any of these treatments was interrupted with moldboard plowing at right angles to the rows, very little surface residue was left. The plots were fairly large (82 x 30 ft) so the authors felt that movement of the fungus between plots was not a major factor in their results. So, what effect did tillage have on Diplodia ear rot? In each of the 3 years of this study, the percentage of ears with Diplodia increased with the greater the amount of surface corn residue in the plot. Statistically, however, the amount of residue accounted for only one-half to three-quarters of the variation in ear rot incidence. My interpretation is that some of the variation in ear rot incidence was due to movement of spores of the fungus from one plot to another (more on this later). For each year of the study, the authors developed a linear equation to relate ear rot incidence to the amount of surface residue. For example, during 1995/96, the year of greatest ear rot, the percentage of infected ears (Y) was related to the amount of surface residue (x), expressed as grams per square meter of ground, by Y = 0.57x + 13.24. What I found interesting about the equation for each year is that it predicted a substantial amount of ear rot when there was no surface residue (i.e., when x = 0). Predicted incidence of ear rot when no residue was present was 13% for 1995/96, 9% for 1993/94, and 11% for 1994/95. In 1995/96 there were actual data points for x = 0. In the other 2 years, the lowest amounts of surface residue were about 2 g/m2, so some extrapolation was required to predict the incidence of ear rot for x = 0, but not much. Over the 3 years of this study, the maximum incidence of ear rot was in the range of 20 to 23%. Thus, although the amount of corn residue had a significant effect on incidence of ear rot, going from no residue to the maximum amount only doubled the incidence, and even with no residue there was still a substantial amount of ear rot. Using data provided by Tony Vyn of Purdue’s Agronomy Department, and an equation provided by Diane Stott of the USDA National Soil Erosion Research Lab at Purdue, it appears that the range of surface coverage among treatments in the South African study was fairly low (about 20% cover for the treatments that left the maximum amount of residue). Thus, amounts of residue than are less than the target for conservation tillage (35%) can evidently result in considerable Diplodia ear rot. This brings me back to the question of movement of spores of Stenocarpella maydis. This fungus produces microscopic spores in small, flask-shaped structures that are embedded in corn residue (mainly stalks, husks, cobs, and kernels). The spores are dispersed by splashing rain and wind-blown rain. I saw one field of corn last year that had almost no corn residue in it. At most, there was one tiny fragment of cob in about every square yard. Soybeans were planted into corn residue to the east of this field. However, well into this corn field, and several hundred yards from the corn residue, Diplodia ear rot was severe. If residue was the source of inoculum, then the spores were clearly dispersed a great distance. Given that Diplodia ear rot was a common disease during the early and mid 1900s, when plowing was common, and that recent research as well as my observations indicate that ear rot can be severe in corn on ground that has little corn residue, it does not seem justifiable to suggest that tillage practices in an individual field or on an individual farm will have a large impact on the risk of Diplodia ear rot. Another consideration (or side of the disease triangle) is hybrid resistance. There are hybrids with good levels of resistance to Diplodia. During a period of general absence of Diplodia (1960s through mid 1990s), some hybrids were developed and released that were susceptible to Diplodia ear rot because breeders were not able to test their lines in the presence of severe Diplodia ear rot pressure. Last year afforded corn breeders the opportunity to evaluate their inbreds, hybrids, and experimental breeding lines. Because Diplodia was widespread in Indiana last year, we can be sure that there will be a lot of the fungus present in residue this summer. Growers, especially those who had a problem with Diplodia last year, should select hybrids for 2001 that have some degree of resistance to this disease. This is a probably a more effective management option than clean tillage. The third variable that will determine whether Diplodia is a problem this summer is weather during silking and early grain filling. If there is a lot of rainfall at this time, the chances of infection are greater.
Risk of Yellow Dwarf in Wheat– (Gregory Shaner)
Doug Johnson, Extension Entomologist at the University of Kentucky, compared temperatures during this past winter to those of 1999-2000 (see Kentucky Pest News, Number 905, Jan 29, 2001 <http://www.uky.edu/Agriculture/kpn/kpnhome.htm>) to predict the activity of aphids on winter wheat and the consequent likelihood of yellow dwarf. He showed that for Princeton, KY the accumulation of Degree Days (DD50) from mid October through January was much less this past year than during the previous year. Initial accumulation of DD50s was about the same during the 2 years, but in 1999-2000, DD50s continued to accumulate during December and January whereas they did not do so during 2000-2001. What does this have to do with yellow dwarf in wheat? Yellow dwarf is caused by either of two closely related viruses: Barley Yellow Dwarf Virus (BYDV) and Cereal Yellow Dwarf Virus (CYDV). (Until recently, these two viruses were considered to be one species, consisting of different serotypes. Two of the serotypes have now been transferred to a “new” virus, CYDV.) These viruses can only be transmitted to wheat by certain species of aphids. If an aphid feeds on an infected plant, it acquires the virus, and then can transmit it to another plant when it feeds there. BYDV and CYDV can only survive in living host plants or in aphid vectors. The extent of infection by these viruses depends on aphid activity and population sizes. During a cool fall and cold winter, aphid activity is low or nil, and spread of these viruses is therefore greatly reduced. Johnson states that although aphids can survive cold temperatures, they are inactive when temperatures are below 48-50°F. In calculating DD50 only positive differences between daily average temperature and 50 degrees are considered. I looked at DD50 accumulations for southwest Indiana for the period October 1 through January 31 for this past winter and for the year before. The pattern of accumulation was similar to what Johnson found for Princeton, KY. During the first half of October, temperatures for both years were similar. During the winter of 2000/2001, DD50s continued to accumulate rapidly for the rest of October, but from the end of November through January, there was no additional accumulation. Conversely, DD50 accumulation stalled out for a few days during mid October of 1999, then continued to increase throughout December. Thus, as in Kentucky, there was a longer period of time during which aphids would have been active during the winter of 1999/2000 than during the winter of 2000/2001. The longer the period during which aphids are active in the fall, the greater the chance that a winter wheat crop will be infected with BYDV or CYDV. The fly-free date for planting winter wheat in the fall was originally devised as a way to reduce risk of infestation by the Hessian fly, but it has proved to be a useful guideline for avoiding other diseases, including yellow dwarf. Planting wheat after the fly-free date reduces the chance that viruliferous aphids will be active. Even in conditions such as occurred last autumn, wheat sown early is a greater risk than wheat sown after the fly-free date. However, aphids probably did not have much opportunity to spread and multiply in wheat sown at or after the fly-free date in the autumn of 2000, so the chance of yellow dwarf infection was probably low. Infection of winter wheat by BYDV or CYDV can occur in the fall or spring. Fall infection is of greater concern. It does much more damage to the plant than spring infection. Fall infection stunts plants, reduces tillering, and stunts roots. Infected plants are more prone to heaving injury, and those that survive yield poorly. Spring infections are more conspicuous than fall infections. Flag leaves are bright yellow or red, and upright. However, the damage is not as great as with fall infection. The weather during February and March has also been too cool for aphid activity, so aphids that are carrying virus or that may move from infected perennial grasses to wheat are probably not doing anything yet. The further along in development the wheat crop is before viruliferous aphids move into it, the less damage there will be from yellow dwarf.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
GMO Issues Facing Indiana Farmers in 2001– (Bob Nielsen) The global debate over genetically modified organisms, specifically transgenic crop varieties, shows little evidence of slowing down. Whether you favor transgenic plant breeding or not, the short term effects on market acceptance for transgenic crops in general are impacting corn and soybean farmers directly. You only have to look at the uproar caused by the contamination of last year’s commercial corn and seed corn production by the Cry9C Bt transgene (approved for animal consumption and industrial use but not human consumption) to realize how quickly the global debate can hit home. As Indiana farmers prepare for the 2001 growing season, what can they expect? Will there be any more unexpected ‘red flags’ regarding the acceptance of currently available transgenic crop varieties? What can farmers do to best minimize the transgenic market risk to their farming operations? First of all, recognize that NONE of the currently available insect-resistant or herbicide-tolerant corn or soybean varieties are CRITICAL for the success of Indiana farmers. European corn borer, the corn pest targeted by Bt corn hybrids, occurs infrequently enough and at sufficiently low levels that the use of Bt hybrids is not economical for most Indiana corn growing situations (Hyde et. al., 1998). Such hybrids are best suited to extremely early or late corn plantings where the risk of injury to the corn borer is greatest. The glyphosate tolerant soybean technology is a very handy weed control tool and often lowers total weed control costs, but cannot be considered critically important for the success of soybean production in Indiana. The same holds true for glyphosate tolerant and glufosinate tolerant corn hybrids. Because these transgenic crop traits are NOT CRITICAL for the success of Indiana farmers, the choice of whether to grow them or not depends primarily on the farmer’s assessment of the uncertainty of market acceptance for such products and/or the available seed supply of alternative non-transgenic varieties. What if a farmer elects not to use transgenic crop varieties, but is concerned about the risk of contamination of his/her grain by transgenic grain? In other words, what are the possible means by which one can end up with transgenic grain interspersed with that produced from a non-transgenic variety? Seed producers face the same challenges of producing pure non-transgenic crop seed as do commercial grain producers. Consequently, most have been reluctant to assure 100% ‘pure’ seed relative to transgene contamination. In late December, the USDA strongly recommended that seed companies sample and test all of their 2001 seed corn lots and all seed parent lines for the presence of the Cry9C Bt transgene because of the hue and cry raised last fall with the discovery of this genetic material in corn flour and products made from corn flour. Any seed lot testing positive for Cry9C will be channeled into feed or non-food industrial use. USDA also recommended that seed companies provide the verification information to customers when customers ask for it. The seed industry has responded to this demand by supposedly testing all seed lots for the presence of the Cry9C Bt transgene. Unfortunately, seed companies cannot guarantee zero presence of Cry9C in any seed lot. The currently available quantitative tests, when used with appropriate sampling intensities, are capable of detecting the presence of the Cry9C protein at the minimum detectable level of no less than about 0.2% with a 99% probability. Every corn grower needs to take reasonable precautions to avoid introducing the Cry9C Bt transgene into the 2001 corn crop. At a minimum, corn farmers should “verify before they buy” and insist on receiving the results from the USDA-recommended seed testing plan for the Cry9C Bt transgene. Ask for the results in writing, keep this documentation for your records, and help to assure the integrity of the 2001 harvest. Additionally, consider saving a sample of seed from each lot of supposed non-transgenic hybrid or variety for purity retesting in the event that you need to re-verify the non-transgenic integrity of a particular seed lot. At a maximum, ask for written assurances for ANY transgene contamination in any non-transgenic corn or soybean variety. Some companies have taken the extra steps to test for any transgene contamination in their non-transgenic hybrid seed lots and are making this information available to their customers. Previous Crop & Variety. Because of the risk of transgenic volunteer corn, any field planted to a transgenic event in 2000 (especially the Cry9C Bt transgene) should not be planted to corn again in 2001. Similarly, be sure to prevent any such volunteer corn in this year’s soybean fields from setting seed. Planting Operation. Let’s say that a farmer has obtained a ‘pure’ supply of non-transgenic seed corn or soybean, but will also be planting some transgenic varieties in 2001. Obviously, then, there will be some potential for seed contamination during the planting operation. The best advice here is to plant the non-transgenic seed lots first, followed by the transgenic varieties. In this way, any seed carrying over from one seed lot to another in the planter will be from non-transgenic to transgenic and not the other direction. Pollen Drift Control. Corn is a cross-pollinating plant species, meaning that pollen freely moves with the wind throughout a corn field and, to a limited degree, outside of the field during the active pollination period. While recent research on the extent of pollen drift strongly suggests that the majority of corn pollen from a field lands within a very short distance from the field, some small percent of pollen will travel a quarter of a mile or further and still be viable. Consequently, pollen drift represents a means of transgene contamination for farmers growing non-transgenic hybrids adjacent to fields of transgenic hybrids. Communication with neighbors is an important aspect of pollen drift awareness. Farmers should find out what corn hybrids will be planted adjacent to their fields of non-transgenic corn, and document the hybrid seed lot information and planting dates. In Indiana, the risk of pollen drift is greatest from fields of corn planted to the southwest of the field in question because of the direction of the prevailing winds in mid-summer. Taking the time to note the dates of pollen shed in your field and adjacent fields will help you determine the relative risk of pollen drift. The risk of pollen drift from neighboring transgenic corn fields may require the harvesting and segregation of a certain amount of corn around the perimeters of a non-transgenic field, certainly no less than 660 feet from the field edge. Corn harvested from those buffer strips should be fed on the farm, or channeled to elevators willing to accept transgenic corn. Harvest Operation. Combines should be super cleaned prior to the start of grain harvest to minimize the risk of any leftover grain from 2000 in the machine. If non-transgenic and transgenic varieties are grown on the same farm, then the sequence of harvesting those fields should follow the FIF-FOF (First-In-Field, First-Off-Field) principle. This means that non-transgenic varieties planted in the field first should be harvested before transgenic ones to avoid transgenic grain commingling with non-transgenic grain from the nooks and crannies of the combine. Handling, Storage & Transport. All grain transport vehicles (trucks, wagons, trailers, grain carts), all grain handling equipment (augers, legs, pits, wet holding bins, dryers) and all grain storage facilities should be super cleaned prior to the start of grain harvest. By following the FIF-FOF principle during harvesting, the post-harvest operations will benefit because non-transgenic varieties can be received, dried and transferred to storage ahead of transgenic varieties. Obviously, transgenic and non-transgenic grain should be stored separately on-farm to avoid grain commingling, and to take advantage of potential premiums for identity-preserved grains in the market place. Assuming that transgenic grain was put into storage last, then emptying storage facilities for transport to market should begin with the transgenic grain in order to avoid an extra cleaning step, and thus, reduce the chance of contamination. However, given that this strategy will depend on a farmer’s marketing plan, all grain transport vehicles and grain handling equipment should be super cleaned prior to every time that non-transgenic grain load-out follows transgenic load-out in order to avoid commingling of grain leftover from the previous handling operation. Guidelines for Corn, 2001:
Guidelines for Soybean, 2001:
Foreign buyers have been buying and appear to continue to be willing to buy glyphosate tolerant soybeans (and meal).
Tips For Corn Planter Tune-ups– (Bob Nielsen) The days are getting longer, the sunshine is becoming more plentiful and temperatures are slowly rising. That can only mean that corn planters will soon be running in fields across Indiana. If you haven’t taken the time to go over your planter or have it inspected and serviced by your local dealer, please take the time to do so before planting begins. Several seed companies plus a number of planter dealers offer planter unit testing using one of several planter test stands on the market. One of the more popular test stands being used is called the Meter Max™, manufactured by Precision Planting™ (<http://precisionplanting.com/>). This type of planter test stand not only measures the accuracy of seeding rate, but can also give you an idea of the uniformity of the seed drop by virtue of the seed dropping onto a horizontal seed belt. Here are some general guidelines and tips for planter maintenance and adjustments.
CALIBRATE THE PLANTER!
Thoughts on Corn Planting Dates– (Bob Nielsen) As the end of March approaches, the anticipation of the start of another planting season is mounting among the faithful patrons of the Chat ‘n Chew Café. Even the excitement of NCAA basketball doesn’t hold a candle to the enthusiasm of the speculation about who will be the first to actually put corn in the ground instead of just pulling the planter around the neighborhood and agitating those trying to be patient about the whole thing. Can you plant too early? Yes. Can you plant too late? Yes. Do you always know ahead of time when the ‘right’ time was to plant? Not always. What are the risks and benefits to early planting of corn? Benefits of Early Planted Corn.
Risks of Early Planted Corn.
Hedging Your Bets.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Greetings From The New Entomology Department Head
It’s my pleasure and privilege to introduce myself. I came from the USDA Cooperative State Research, Education, and Extension Service where I worked as the National Program Leader for Biological Control and Applied Ecology for the past 2 years. Prior to that I was a scientist and team leader with the International Institute of Tropical Agriculture (IITA) in Africa where I lived in Nigeria for 5 years, Benin for 9 years, and in the Netherlands for a year while on sabbatical leave at the University of Amsterdam. I came to Africa from California where I worked and studied at the University of California, Berkeley from 1972 to 1983 including a 2 year study abroad experience at the University of Nairobi, Kenya in 1974 and 1975. I have a bachelor’s degree in biology, masters in zoology, and Ph.D. in entomology all from U. C. Berkeley. My academic background is in invertebrate zoology, entomology and applied ecology with an emphasis in systems analysis and crop protection. My research interests and experience cover a wide range of plant protection areas including pest diagnosis, taxonomy, yield-loss assessment, population ecology, intervention technology development, implementation and evaluation in agroecosystems. My pest management philosophy has its roots in biological control - the foundation of sustainable plant protection. My immediate plans are to get to know the staff in the Department, learn the culture and the procedures of the School of Agriculture and the University, and develop a strategic plan for Entomology during the first year. This will be an ambitious agenda given all the other responsibilities we have in the Department, but one that is necessary and promises significant returns if properly implemented. These are exciting times for entomology and the life sciences in general. The hiring freezes and related financial crises of the 1990s are now behind us. More money is being invested into both fundamental and applied R&D across the board with universities in the lead. Technological advancements in computing and molecular biology are at the forefront of what I believe will be an exciting new horizon for the life sciences.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||