Pest & Crop Newsletter
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Black Cutworm Coming On Strong, Scout Now– (John Obermeyer, Rich Edwards, and Larry Bledsoe)
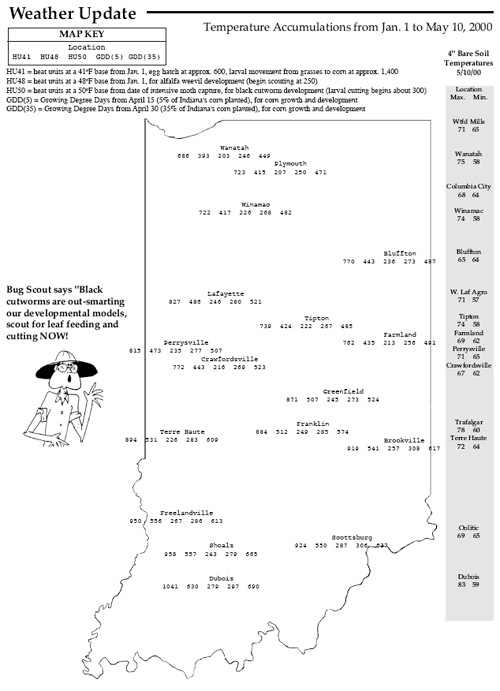
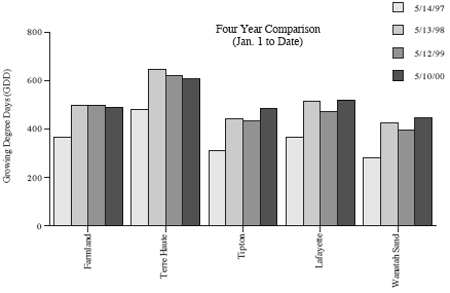
First thing Monday morning (May 8), Gibson county producer Mike Hirsch reported that black cutworm and leaf feeding had been observed and several fields treated over the weekend. Soon to follow was Tony Halter with Pioneer Hi-Bred in southeastern Indiana, informing us of several corn fields being damaged, some severely. Ron Blackwell, IPM survey specialist, traveled to southwestern counties on Wednesday to assess the situation and confirmed the potential damage brewing. Just like last year, the early arriving moths (February and March) and subsequent flights apparently have survived, established in weedy fields, and are now feeding. The underlying theme is that larger cutworms (up to 6th instar found) are cutting corn and smaller ones are feeding on the leaves. This leaf feeding appears minor, but it will soon become cutting as the cutworms grow. As this newsletter was being put to bed, Randy Schelle from Fayette County called to report fields being damaged and rescue treatments being applied. NOW is the time to be scouting! As we graphed last week in this newsletter, large numbers of black cutworm moths have been captured by our pheromone trap cooperators. This certainly set the stage for, but did not necessarily guarantee, economic damage to Hoosier cornfields. Black cutworm moths are particularly fond of winter annuals, such as chickweed and mustards, to lay eggs on. Fields that were showing lots of green a month or two ago, are at highest risk for cutworm damage. This includes fields that were treated at planting with a soil insecticide. Remember, corn and soybean are not the preferred food of the black cutworm. It just so happens that it is normally the only plant remaining by the time larvae have hatched and weeds have been killed. Scout by inspecting 20 consecutive plants in each of 5 areas of a field (100 plants) for cutworms and feeding activity. Count and record the number of plants cut or damaged and determine the percentage of plants affected. Also collect black cutworm larvae and determine the average instar stage. While sampling, also record how many leaves are fully unrolled (the collar of the leaf is visible on a fully unrolled leaf). Control of black cutworm may be necessary if 3 to 5% of the plants are damaged and the average larval instar is from 4 to 6. Use the following management guidelines and instar guide. Suggested foliar insecticides for control of economic infestations are listed below.
Corn Flea Beetle Making Their Presence Known– (John Obermeyer, Rich Edwards, and Larry Bledsoe)
There is no doubt that the mild winter has contributed to the high numbers of corn flea beetles being observed. This tiny (1/16”), shiny black beetle feeds on corn leaves by stripping off the top layer of plant tissue. This feeding leaves gray to brown lines or “tracks” etched on the leaf surface. Heavily infested plants may appear gray as their leaves shrivel and die. On seedling dent corn, control may be necessary if 50% of the plants inspected show severe corn flea beetle feeding damage (plants begin to look silvery or whitish, or leaves begin to die), approximately 5 or more corn flea beetle per plant are found, and poor growing conditions are causing slow corn growth (e.g., cool temperatures, dry soils, herbicide damage). Normally, once a corn plant reaches the growth stage V5, it is no longer susceptible to significant corn flea beetle damage. Therefore, sampling for corn flea beetle typically will not be necessary once the plants have 5 leaves. Corn flea beetle may also transmit the bacterium that causes Stewart’s wilt as it feeds. This can be a serious problem, especially on sweetcorn and seed corn inbreds. In sweetcorn, the disease may result in ears that are smaller than normal, or some infected plants may die. In seed production fields, severe leaf blight may cause lightweight chaffy ears, plus increase the likelihood of stalk rots. The beetles alone are seldom severe enough to kill plants although in combination with the disease, such as noted above for sweetcorn, they may. In seed production fields where highly susceptible inbreds are utilized, treatment is probably justified if corn flea beetles are noted.
Armyworm in Corn and Wheat– (John Obermeyer, Rich Edwards, and Larry Bledsoe)
Spring flying armyworm moths prefer to lay their eggs on dense grassy vegetation (e.g., wheat and grass cover crops) and the hatched larvae will feed on both corn and wheat. Corn - Corn that has been no-tilled into or growing adjacent to a grass cover crop (especially rye) should be inspected immediately for armyworm feeding. Hatched larvae will move from the dying grasses to emerging/emerged corn. Armyworm feeding gives corn a ragged appearance, feeding from the leaf margin toward the midrib. Damage may be so extensive that most of the plant, except the midrib and stalk, is consumed. A highly damaged plant may recover if the growing point has not been destroyed. If more than 50% of the plants show armyworm feeding and live larvae less than 1-1/4 inches long are numerous in the field, a control may be necessary. Larvae greater than 1-1/4 inches will soon be pupating and controls are futile because the damage has already been done. If armyworm are detected migrating from border areas or waterways within fields, spot treatments in these areas are possible if the problem is identified early enough. Wheat - Examine plants in different areas of a field, especially where plant growth is dense. Look for flag leaf feeding, clipped heads, and armyworm droppings (excrement) on the ground. Shake the plants and count the number of armyworm on the ground and under plant debris. On sunny days, the armyworm will take shelter under crop residue or soil clods. If counts average approximately 5 or more per linear foot of row, the worms are less than 1-1/4 inches long and not parasitized or diseased, and leaf feeding is evident, control may be justified. If a significant number of armyworm are present and they are destroying the leaves, or the heads, treat immediately.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
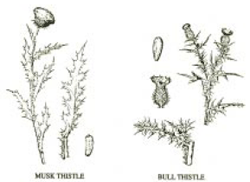
Bull and Musk (Biennial) Thistle Control In Perennial Grass Crops– (Merrill A. Ross) Musk thistle and bull thistle are biennials causing major problems in perennial grass crops including pastures, roadsides, conservation reserve and fence rows. Biennials require portions of two growing seasons to reproduce. They grow from seed the first season as a rosette (a taproot with a cluster of leaves on the soil surface). The rosette overwinters and cold causes the rosette to send up a flowering stalk the next season and produce seed. Once seeds mature, the plant dies. Destruction of rosettes prior to flowering is an effective means of preventing seed formation and subsequent spread. Management of pastures infested with biennial thistle requires special consideration. Since biennial thistles reestablish from seed which is dispersed by wind, it is helpful to prevent seed formation adjacent to pastures. Also it may take two or more years of excellent control before seeds are reduced to the point that allows for legume reestablishment for pasture improvement. One year of poor thistle control will result in having to start the control program over. Most of the herbicides used for control of bull and musk thistle also kill pasture legumes. Spot spraying individual plants or patches rather than broadcast spraying the entire pasture also spares the legumes.
Musk thistle normally initiates flower stalks in early May and reaches full flower in early June. Seed production is prolific and usually completed in mid to late June. Rosettes reestablish nearly any time during the growing season. Some rosettes may be three or four feet in diameter by late fall. Musk thistle is extremely aggressive. Bull thistle normally initiates flower shoots in July and reaches full flower in August. Seed production is usually completed in late summer. Rosettes reestablish during summer and early fall. USE OF POSTEMERGENCE HERBICIDES FOR CONTROL OF BIENNIAL THISTLES The best time to treat biennial thistles with herbicides is in late fall or early spring when the rosettes are present but before flowering stalks are initiated. Musk thistle and bull thistle plants with seed stalks are more difficult to kill than the rosettes. Thistle rosettes need to be treated when they are actively growing and not under drought stress. The younger the rosette, the more susceptible it is to herbicide. FOLIAR TREATMENTS FOR SELECTIVE REMOVAL OF BIENNIAL THISTLE FROM PASTURE GRASSES One properly timed treatment per year should prevent seed formation. Several herbicides provide good to excellent control of thistle rosettes. Fall treatments should be made late enough to kill all rosettes germinated before winter. Late germinating rosettes that establish after early fall herbicide applications could flower the next growing season. Early spring treatments should kill all overwintering rosettes and those rosettes germinating later (spring and summer) should not produce seed until the following year. 2,4-D (Numerous trade names. Some of the more recognizable include: Esteron 99, Weedone 638, Weedar 64, Formula 40 and HiDep). 1 to 1 1/2 qt./acre. BANVEL/CLARITY (dicamba) 1 pt./acre. STINGER (clopyralid) 1/3 to 2/3 pt./acre. Spot treatment only or when Canada thistle is present. Forage legumes will also be killed. Legumes cannot be reseeded into treated areas for 12 months following treatment ALLY (metsulfuron) 1/4 to 1/2 oz./acre. Mixing with 2,4-D frequently reduces injury to pasture grasses. CROSSBOW (triclopyr + 2,4-D) 2 qt./acre where woody species are present. Plateau (imazapic) 8 to 12 oz./acre for Conservation Reserve, and wild flower establishment and other non-cropland only uses. Doses of imazapic PLATEAU that control thistle tend to severely injure tall fescue. TORDON (picloram) long soil-residual selective control in permanent pastures where brush control is also desired. FOLIAR TREATMENTS FOR NONSELECTIVE REMOVAL OF BIENNIAL THISTLE FROM PASTURE GRASSES ROUNDUP/TOUCHDOWN (glyphosate) 1to 2 qt./acre or 1 to 2% solution GENERAL CHARACTERISTICS OF CANADA THISTLE Canada thistle is a cool season perennial which spreads by seed and vegetatively by creeping roots. In Indiana, Canada thistle normally initiates growth in spring, reaches the flower-bud stage the first week in June, and full flower about the third week of June. Seed production is usually completed in early July. Undisturbed plants tend to become inactive during hot weather (July and August). Then new shoots emerge during September and survive into November. The growth on Canada thistle in late September and October helps restore underground food reserves. The best time to treat Canada thistle with foliarly applied herbicides which move down through the plant is in early June after the first flower buds are formed and before the first flowers open and/or on fall regrowth during September and early October. Mowing, tillage, or herbicide application can alter the normal growth pattern and provide regrowth suitable for spraying. Creeping roots provide stored food and numerous underground buds to depths well below the plow layer. Removal of shoots and severe damage to established plants stimulate new growth from underground buds. It is the buds on the creeping roots of established Canada thistle plants which largely account for re-establishment after attempts at control. Buds on creeping roots can generate new shoots a year or more after top-growth has been destroyed. Seed is produced when patches with male flowers are in close proximity to patches with female flowers. While young seedlings are not competitive in most crops, they provide a source of re-establishment and take on perennial characteristics after about two months. Seeds can survive burial in the soil for 20 years or more.
CONTROL PRACTICES FOR CANADA THISTLE I. SHADING — Canada thistle is susceptible to shading. Thus, it grows most vigorously when no competing vegetation is present. Small grains compete less effectively against Canada thistle than most other agronomic crops grown in Indiana. Shade from a vigorous crop often provides much of the control needed to keep Canada thistle in check during the growing season. Conditions which favor rapid closure of crop canopies (good stands, adequate fertility and narrower row spacing) maximize the control provided from a competing crop and compliments other control practices (e.g., herbicides). II. HERBICIDES — Herbicides for Canada thistle can be grouped as follows: 1) those applied to the foliage and which move down through the plant causing substantial injury to the creeping root systems, 2) those applied to the foliage and which move down through the plant causing partial (and variable) injury to the creeping root systems, 3) those applied to the foliage and which do not move down through the plant and can only result in shoot kill or inhibition (no injury to the creeping root systems can be expected), and 4) long residual herbicides which can be appropriate for permanent pastures, bare soil sites and other non-crop situations. A. Foliarly applied herbicides which move down through the plant and cause substantial injury to the creeping root systems. One properly timed application may provide 50 to 90 percent control on shoot regrowth 6 months to a year later. At least one additional application made to regrowth 3 to 12 months later is needed to provide adequate stand reductions to provide long term control. Canada thistle can be treated after heavy frost and light freezes if the leaves appear not to be injured. B. Foliarly applied herbicides which move down through the plant and cause partial (and variable) injury to the creeping root systems. These herbicides provide suppression of thistle shoot growth and may provide some long-term control if applied two or more times a year in conjunction with a competitive crop. Conditions needed for good performance of foliarly applied downwardly mobile herbicides on Canada thistle: 1 - Adequate soil moisture from the soil surface well into the subsoil. C. Foliarly applied herbicides which DO NOT move down through the plant and thus result only in shoot kill or inhibition. No injury to the creeping root systems can be expected. These provide shoot kill and/or subsequent suppression in a competing crop but not much long-term effect when used alone. D. Long residual herbicides which can be appropriate for bare soil sites (these should not be used on cropland). HERBICIDE SUGGESTIONS FOR THE 2000 GROWING SEASON Selective control with glyphosate tolerant crops Glyphosate ROUNDUP ULTRA in conjunction with glyphosate tolerant (Roundup Ready) crops — Soybeans are the easiest to use. Plant glyphosate resistant ROUNDUP READY soybeans. Then apply 1 qt/acre of glyphosate ROUNDUP ULTRA (or other labeled glyphosate herbicide product) over the top of soybeans and thistle when Canada thistle reaches six or more inches tall. Follow up as appropriate on thistles which recover. Selective control with normal germplasm crops Between crop applications of glyphosate — ROUNDUP (TOUCHDOWN) at 1 to 1 1/2 qt/acre applied in late may or early June after flower buds are formed and before flowers open and/or on fall regrowth in late September and early October. Glyphosate treatments followed by a crop which provides good shade and a selective herbicide to remove the shoots of thistle plants which escape can result in reductions of thistle approaching 50 to 90 percent a full year after treatment. Split applications of two 1 qt applications of glyphosate (3 to 12 months apart) should prove more effective than a single 2 qt application. Corn — A clopyralid and flumetsulam combination sold as HORNET is less costly and next most effective to STINGER for selective control of Canada thistle in normal corn. Soybeans (at leaf drop) preharvest with glyphosate ROUNDUP 1 qt/acre. Grass sods for pasture, conservation reserve and ground cover — Clopyralid STINGER at 2/3 pt/acre. High cost of chemical usually limits use of STINGER to spot treatments. One or more applications per season in established perennial grass cover should be applied in late May or early June as flower buds (8 to 12 inch stems) are formed and before flowers open. Canada thistle should be retreated when subsequent regrowth reaches 6 to 12 inches in height. A treatment on fall regrowth should be included. One or more treatments per season will be needed several consecutive years for complete control. Grass sods are less competitive with Canada thistle than corn or soybeans. Selective top removal (and sometimes more) in crops THIS IS THE MINIMUM THAT CAN BE DONE TO MEET THE INDIANA WEED LAW REQUIREMENTS) These herbicides are used to selectively remove or suppress the tops of Canada thistle in crops which will grow rapidly enough to provide a closed canopy and shade the ground before new thistle shoots recover (Soybeans in drilled plantings or corn in 30 inch rows). None of these herbicide treatments should provide adequate control of Canada thistle unless the shade from a competing crop is obtained. Thistle can be expected to re-grow and recover in the fall following crop maturation and harvest. Corn (tall varieties in 30 inch rows) — Dicamba BANVEL/CLARITY/DISTINCT, 2,4-D (numerous trade names), primisulfuron BEACON, bentazon BASAGRAN, applied postemergence at doses suggested for selective control of broadleaf weeds in corn. Combinations of these herbicides [or mixtures of atrazine with one of these herbicides either as prepackaged mixtures (e.g., MARKSMAN, LADDOK) or as tank mixtures] can also be used. Wheat (Wheat is much less competitive with Canada thistle than corn and soybeans) Clopyralid STINGER, dicamba BANVEL/CLARITY, 2,4-D, MCPA, or thifensulfuron + tribenuron EXPRESS HARMONY EXTRA after wheat is tillered and at doses and timings suggested on product labels. Solid Stand soybeans (top kill only) — Postemergence herbicides applied at higher labeled doses include bentazon BASAGRAN, acifluorfen BLAZER or TACKLE, chlorimuron CLASSIC, lactofen COBRA, or combinations of two herbicides (e.g., bentazon + acifluorfen or bentazon + imazethapyr PURSUIT). Imazapic PLATEAU (for Conservation Reserve, and wild flower establishment and other non-cropland only uses), has provided longer term control than just top removal in our limited testing. Doses of imazapic PLATEAU (8 to 12 oz/acre) that control thistle tend to severely injure tall fescue. selective control in established tree crops — Applications of glyphosate ROUNDUP 1 1/2 qt per acre directed underneath foliage and green bark of established trees and over Canada thistle 10 to 18 inches tall should be made in late spring or on fall regrowth. Canada thistle is at the correct height about June first (by June 20 thistle is 36 to 48 inches tall in full bloom) and in the fall (September and early October). Removal of low growing tree branches will make directed applications easier and less likely to injure trees. Roundup should not be applied to green or freshly injured stems. Two or more applications at 3 to 12 month intervals will be required to provide adequate control. Canada thistle blooms heavily in late June. An effective control program must include treatments for any plants growing in the spring. Clopyralid STINGER is labeled for selective control of Canada thistle in some conifers. Cost of treatment limits the use to spot treatment. non-selective site preparation for perennial crops— Two applications of glyphosate ROUNDUP/TOUCHDOWN 1 1/2 qt per acre at 3 to 12 month intervals (fall/spring, spring/fall, spring/spring, or fall/fall) made to Canada thistle plants 10 to 18 inches tall under good growing conditions should allow establishment of perennial crops (turf, pastures, Christmas trees, and nurseries) almost free from competing Canada thistle plants. This approach requires advanced planning and herbicide applications well ahead of crop establishment. An effective control program must include treatments for any plants growing in the spring, since Canada thistle blooms heavily in late June. For no soil-residual (non-selective) Glyphosate ROUNDUP/TOUCHDOWN AT 1 TO 2 qt/acre TWO APPLICATIONS PER SEASON; one in June after flower buds are formed and before flowers open and a second on regrowth when it reaches 8 to 10 inches tall mid-season or 6 to 8 inches tall in September and early October. Applications of 1 to 1 1/2 qt/acre applied twice (initially and then on regrowth) will be more effective than a 2 to 3 qt/acre single dose. Long soil-residual non-selective control WHERE NO VEGETATION IS DESIRED. Before any of the following herbicides are applied, both the site and herbicide chosen should be evaluated for potential harmful environmental consequences such as soil erosion, damage to nearby off-target vegetation and contamination of ground water. Chlorsulfuron TELAR, picloram TORDON, prometon + sodium-chlorate + sodium-metaborate PRAMITOL P, and imazapyr ARSENAL.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fusarium Head Blight (Scab) of Wheat– (Gregory Shaner)
Although the spring has generally been dry, recent rains may promote development of Fusarium head blight, or scab, of wheat. Fusarium head blight is caused by any of several fungi in the genus Fusarium. In the Corn Belt, the species Fusarium graminearum appears to be principally responsible. Fusarium graminearum is also a pathogen of corn, causing Gibberella ear rot and stalk rot. The fungus overwinters in Indiana in residue of wheat or corn plants that were infected the previous year. With the far greater amount of corn residue compared to wheat residue in Indiana at this time of year, corn is thought to be the main source of inoculum. Inoculum (the infective form of the fungus) consists of airborne ascospores produced on crop residue. Moisture seems to play two roles in the epidemiology of wheat scab. It promotes the development and release of ascospores on corn residue and it provides the conditions necessary for these spores to infect wheat. The fungus infects wheat flowers directly. Anthers, which extrude shortly after pollen is shed, are thought to be the most frequent site of infection, but spores can infect the wheat ovary and may even infect directly through the floral bracts (glumes, lemmas, paleas). The fungus progresses into the developing wheat kernel, where it largely replaces the endosperm. The fungus also moves up and down the rachis from the point of infection and invades other kernels in the head. An infected kernel is shriveled, the degree depending on how early in its development it was infected. In addition to reducing yield and test weight, F. graminearum produces toxins (mainly deoxynivalenol, or DON) in infected grain. Although we do not have a clear understanding of the relation between weather and development of scab, it is known that long periods of wet, humid weather while wheat is flowering are conducive to the disease. This spring we have not had the almost constant drizzling rains that characterized the wheat flowering period in Indiana during 1996, when scab was extremely severe, but areas of the state have had rains almost every day during wheat flowering. It is quite possible that some scab will develop in these areas.
Leaf and Glume Blotch of Wheat– (Gregory Shaner)
So far, there appears to be much less leaf blotch of wheat than is normal for Indiana. Leaf blotch is caused by either of two fungi: Septoria tritici or Stagonospora nodorum. Symptoms of Septoria blotch and Stagonospora blotch are similar and accurate diagnosis requires examination of infected tissue under a microscope. The spores produced by each fungus are distinctive and provide the easiest means of determining which pathogen is responsible for the symptoms. A single plant, or even a single leaf may be infected by both pathogens.
Tan Spot of Wheat– (Gregory Shaner)
I have seen tan spot on wheat plots in Tippecanoe and Jennings Counties and on some samples submitted to the PPDL. Symptoms described over the phone have also sounded like tan spot in some cases. Tan spot is normally a minor leaf disease of wheat in Indiana, but it can be confused with Septoria or Stagonospora blotch. Symptoms consist of dead, tan lesions on leaves. Spots are usually more abundant on lower leaves, and symptoms progress up the plant over time. A fungus (Pyrenophora tritici-repentis) unrelated to the fungi that cause Septoria causes tan spot and Stagonospora blotches. One way to distinguish tan spot from Septoria or Stagonospora blotch is that tan spot lesions do not contain small black specks (pycnidia). However, absence of these specks is not conclusive because they are often difficult to see in lesions caused by Stagonospora. Accurate diagnosis requires microscopic examination of leaf tissue. The tan spot fungus seems to be well established and common in Indiana, but the disease is usually not a problem. The only time I have seen severe tan spot is when wheat is planted directly into stubble of a wheat crop from the previous year, or when there is wheat stubble adjacent to a field of wheat. In this case, tan spot is usually confined to the part of the wheat field nearest the stubble. The spores of the tan spot fungus are relatively large (although still microscopic) and perhaps are not carried far by wind before they settle out. Unlike Septoria and Stagonospora, which are favored by long periods of wet weather, Pyrenophora requires only a few hours of leaf surface wetness for spores to infect the wheat leaf. I don’t anticipate a major problem with tan spot, but in scouting fields, this disease should be kept in mind as a possible reason for leaf spotting.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The Root of the Matter– (Bob Nielsen)
Corn is a grass and has a fibrous type root system, as compared to soybeans or alfalfa which have tap root systems. Successful establishment of the corn plant’s root system helps ensure successful establishment of the crop itself. Stunting or restriction of the root system, especially in young plants, can easily stunt the entire plant’s development. To better understand rooting problems, it is important to understand that root development in corn can be characterized by root position relative to the seed. The Seminal (Seed) Root System Seminal roots originate near the seed and are comprised of the radicle & lateral seminal roots. This initial seminal root system anchors the young plant and absorbs water and nutrients for the first two to three weeks. Seminal roots cease new growth shortly after the coleoptile emerges at the soil surface. A young corn seedling depends primarily on the energy reserves of the kernel until permanent (nodal) roots develop. Within a few days after emergence of the coleoptile and first leaves from the soil, a second root system, the nodal roots, begin to develop from the crown or growing point. If damage occurs to seminal roots or the mesocotyl before nodal roots become established, stunting or death of the plant will occur. Such damage includes salt injury from excessive rates of starter fertilizer, seedling blight, herbicide injury and insect feeding damage. Nodal (Or Permanent) Root System Nodal roots begin to elongate from the coleoptile crown shortly after growth stage VE and are distinctly visible by growth stage V1. An individual set of roots forms at each stalk node below-ground plus one or more above-ground nodes. By growth stage V6, the nodal roots are the main root system of the plant. Four stalk nodes usually comprise the ‘woody’ triangle at the bottom of a corn stalk. The internode above the fourth node elongates about 1/2 inch, above which is found the fifth node (still below or just at the soil surface). Consequently, five sets of nodal roots will usually be detectable below ground (one set for each below ground stalk node). Elongation of the internode above the fifth node ‘pushes’ the sixth node above ground. Continued elongation of subsequent stalk internodes will result in higher and higher placement of the remaining stalk nodes. Additional sets of nodal roots that form at above ground stalk nodes are usually assigned the ‘fancy’ name of brace roots, but are functionally identical to those nodal roots that form below ground. If surface soil conditions are suitable (moist and not excessively hot), brace roots can successfully enter the soil, proliferate and effectively scavenge the upper soil layer for water and nutrients.
Can Corn Survive Leafing-Out Underground?– (Peter Thomison, Ohio State University, Hort. & Crop Science Dept.) (Originally published in Ohio C.O.R.N. Newsletter, 8 May 2000)
When certain unfavorable environmental conditions (especially dry, cloddy soils) occur, germinating corn seedlings may start unfurling leaves below ground. This premature leafing out may also be associated with twisted shoots (coleoptiles). When the problem is widespread across a field, replanting is often necessary. Normally the coleoptile is pointed and quite stiff, it can spike or push its way through soil during emergence. However, in a cloddy field where soil coverage of seed is poor and irregular, sunlight can reach the seedling and induce leaf emergence beneath the soil surface. Other factors (or combinations of factors) can also result in abnormal unfurling symptoms. Heavy rains after planting can cause a hard crust which makes emergence of small seedlings very difficult. As a result, bending and twisting of the seedling below the crusted layer often occurs. Planting the seed too deep, which may cause poor germination and emergence, may also result in premature unfurling of the corn. Certain herbicides such as Lasso and Dual, and the premixes that contain their active ingredients, can show similar symptoms (i.e., twisting, abnormal growth) when excessive rates are applied pre-emergence. Besides excessive rates, improperly closed seed furrows can allow the pre-emergence herbicide to come in direct contact with the seed. Excessive soil insecticide dosage or in-furrow placement of insecticide phytotoxic to seed can also cause twisting sprouts and abnormal leaf expansion underground. In addition, anhydrous and aqua ammonia fertilizer injury has been associated with these symptoms. Certain corn hybrids are also more prone to premature unfurling during emergence. Corn seedlings that exhibit abnormal unfurling symptoms during emergence will be unable to penetrate any but the loosest soil even if the crust is broken mechanically or softened by rain. When the coleoptile ruptures below ground, the leaf that is exposed is quite wide, no longer pointed or rigid, and cannot push its way to the soil surface. Instead it spreads out, stays yellow and eventually dies. Prompt treatment with a rotary hoe, weeder, spike-tooth harrow or cultipacker may help break the crust and improve emergence. To minimize poor seedling emergence due to unfurling below the soil surface, watch for cloddy seedbeds, open seed furrows, and crusting surface soils after rains. Also, check planting depth periodically and adjust accordingly during the planting operation, and monitor herbicide and soil insecticide rates.
Growing Points of Interest– (Bob Nielsen)
There is something about the rumble of thunder, sheets of driving rain and a few hailstones that makes one curious about the ability of corn to recover from early season damage. When corn is damaged early in the growing season, growers are sometimes faced with the decision of whether or not to replant the field. One of the most important, and most difficult, steps in making a replant decision is estimating the surviving plant population in the field. Corn is remarkably resilient to above-ground damage early in the season, yet growers often underestimate the recovery potential of a damaged corn field. As a result, much replanting is unnecessarily performed each year. Use my replant publication (AY-264) to estimate yield and dollar returns to corn replanting. The health and condition of the corn plant’s growing point region plays a major role in determining whether a damaged corn plant will recover or not. A damaged plant with a healthy, undamaged growing point (apical meristem) will survive. Damage to the growing point area will either kill the plant or severely stunt its recovery. Prior to V6, a plant is relatively immune to above-ground damage from ‘single event’ damage by frost, hail, cutworm, sandblasting, anhydrous ammonia burn, 28% N solution burn, and paraquat drift. However, repeated injury to young plants may stunt a plant’s development severely enough to eventually kill the plant even though the growing point was technically not injured. While corn younger than V6 can tolerate a fair degree of above-ground damage to leaf tissue by frost, lethal cold temperatures (32°F or less for several hours) can ‘penetrate’ the upper soil surface and damage or kill the growing point of a young corn plant. Corn younger than V6 is also susceptible to below-ground damage from soil insects, disease and flooding. Damaged corn fields need to be left alone for several days after the damage occurs to give them some time to initiate recovery. Recovery from the whorl will appear within 3 to 10 days, depending on temperature and soil moisture. Warmer temperatures and adequate soil moisture encourage rapid recovery, while cooler temperatures and/or drought stress restrict the rate of recovery. The stalk tissue near the growing point region should remain firm and yellowish-white. Injury occurring close to the growing point may alter normal hormonal activity and cause deformed regrowth. Given sufficient time, surviving corn plants should be showing new leaf tissue expanding from the whorls, while dead corn plants will still look dead.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||