USDA-NIFA Extension IPM Grant
Pest & Crop Newsletter, Entomology Extension, Purdue University
Winter Temperatures, Corn Flea Beetle Survival, and Potential for Stewart’s Wilt - (Christian Krupke, John Obermeyer, and Kiersten Wise)
• Corn flea beetle winter survival is expected to be low in northern and central Indiana.
• Moderate survival is expected for southern Indiana, higher in the Ohio River valley.
• Corn flea beetle is a vector of Stewart’s bacterial wilt and leaf blight of corn.
• Seed applied insecticides generally prevent early corn flea beetle feeding.
Corn flea beetle is a sporadic corn pest in Indiana and has had little impact of recent years. Still, winter temperatures in regions where beetles were abundant last season will determine if there is cause to be concerned this spring for susceptible inbreds and hybrids. This is especially important since this insect transmits the bacterium that causes Stewart’s disease in corn. The severity of the disease correlates with last season’s beetle abundance and this winter’s temperatures. This is because the Stewart’s wilt bacterium survives in the gut of the overwintering beetles and depends upon the beetle to infect corn. Warmer temperatures result in higher beetle survival, and therefore a greater potential for Stewart’s disease.
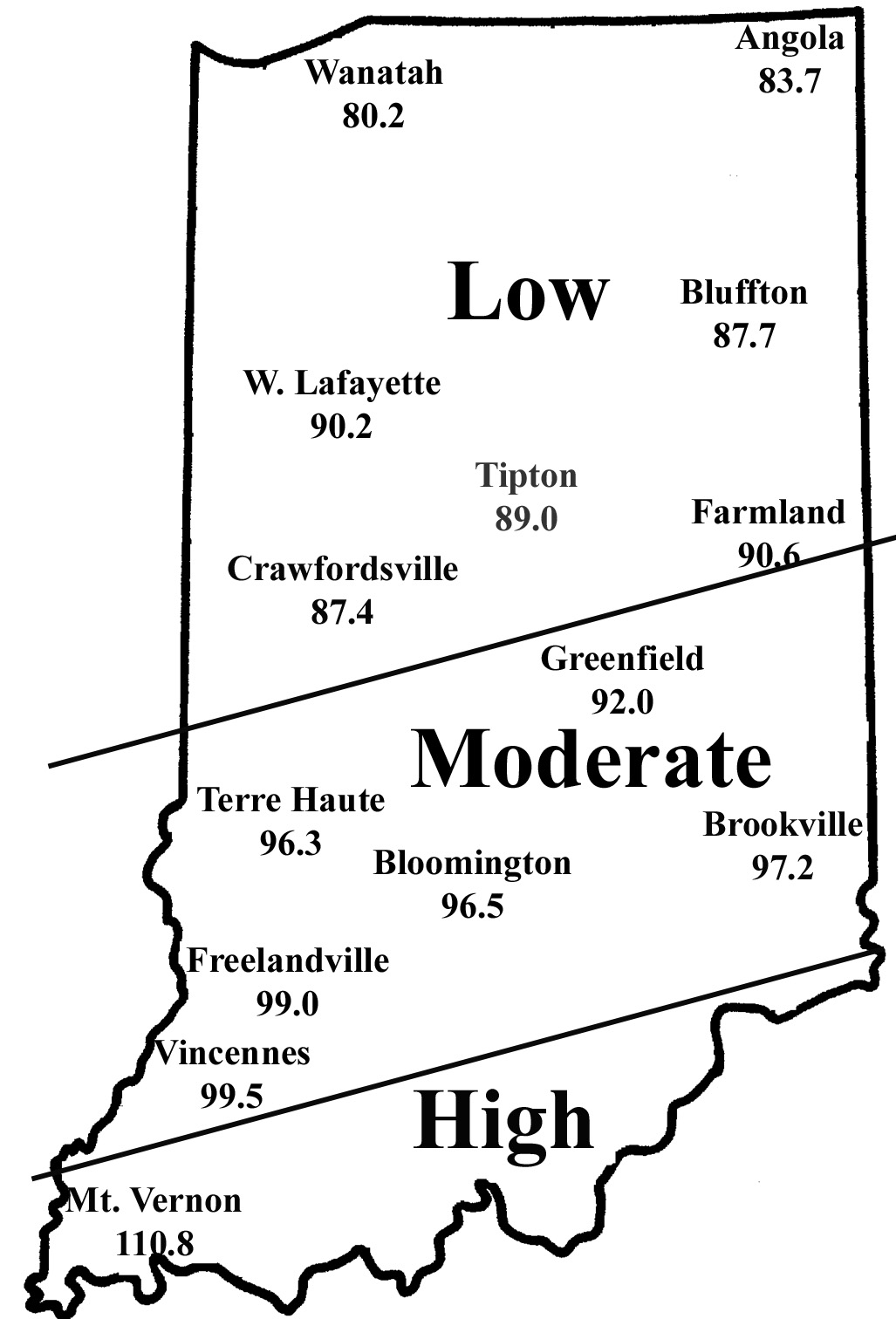
To determine the potential severity of Stewart’s disease, add the average daily temperatures for the months of December, January, and February. If the sum is below 90, the potential for disease problems to develop is low. If between 90 and 100, moderate disease activity is a possibility. Sums above 100 indicate a high probability that beetles will survive the winter and vectoring of Stewart’s disease will occur. To help you better gauge the potential for corn flea beetle activity in your area (and the potential severity of the threat of the disease) in 2013, we have created the state map shown below. According to the temperature model, there is low probability of corn flea beetle activity and subsequent disease in northern and central Indiana, moderate in areas south of Interstate 70, and higher south of US 50.
This temperature model for corn flea beetle has been in use for many years and has been fairly accurate in predicting the activity of this pest the following spring. However one inherent flaw is that the model is based on ambient air temperatures, not temperatures under leaf litter and grass clumps where this pest is actually overwintering. If snow cover is present, this provides an insulating blanket for the insect, and may protect some beetles from winterkill. Even with this “disclaimer” statement, we think the 2012/2013 winter was cold enough to have negatively impacted overwintering beetles in most of Indiana. Also, flea beetle numbers have been low statewide for the last several years.
As for the disease, there are two phases of Stewart’s wilt: a seedling wilt phase and a leaf blight phase. In the wilt phase, plants wilt rapidly, usually at an early stage of growth. Leaves emerging from the whorl of infected plants are often the first to wilt. Internal tissues at the growing point are discolored or hollowed out. Faint green to yellow streaks containing corn flea beetle feeding marks are visible on one or more leaves. If stalks of wilted plants are cut, it may be possible to see yellow beads of bacteria ooze from the vascular tissue. Sweet corn hybrids are especially susceptible. Some dent corn inbreds, and occasionally dent corn hybrids, and some popcorn lines are susceptible as well. Dent corn hybrids rarely show symptoms of the wilt phase after growth stage V5.
The leaf blight phase can occur at any time during the growing season, but often does not appear until after tasseling. Lesions are typically long and narrow, with greenish-yellow streaks and irregular or wavy-margins. Lesions will become straw-colored, and infected leaves may die prematurely. Hybrids with resistance to Stewart’s wilt may have smaller lesions that are limited to the tissue surrounding the feeding site of the beetle. These lesions can be confused with the fungal diseases gray leaf spot or northern corn leaf blight. Stewart’s wilt is also commonly confused with another bacterial disease, Goss’s wilt. One way to differentiate between these diseases and Stewart’s wilt is to look for the beetle feeding scars associated with Stewart’s wilt.
Management decisions made now should be based on the corn’s susceptibility to the disease and anticipated risk. With virtually all corn seed treated with insecticide (Poncho or Cruiser), the only decision to be made is whether a low or higher insecticide rate should provide sufficient protection. Those decisions have likely been made months ago, and even the lowest available rates of seed treatments (Poncho 250) are expected to provide protection from emergence to 2-leaf corn, whereas the higher rate (eg. Poncho 1250 and Cruiser 1.25, also called the “rootworm rate”) should protect corn through the 5th leaf stage. If seed-applied insecticide is not an option, broadcast application of foliar insecticides at the time when corn spikes should provide 7-10 days of residual protection from beetle feeding.
Expected Flea Beetle Winter Survival
Expected Flea Beetle Winter Survival
| Monthly Average Temperatures by Location Disease |
|||||
| Site | Dec. | Jan. | Feb. | Sum | Disease Threat |
| Angola | 33.5 | 26.7 | 23.5 | 83.7 | Low |
| Wanatah | 33.1 | 24.0 | 23.1 | 80.2 | Low |
| Bluffton | 32.0 | 28.3 | 27.4 | 87.7 | Low |
| W. Lafayette | 33.0 | 29.0 | 28.2 | 90.2 | Low |
| Tipton | 32.2 | 28.9 | 27.9 | 89.0 | Low |
| Farmland | 32.5 | 30.3 | 27.8 | 90.6 | Low |
| Crawfordsville | 30.7 | 28.6 | 28.1 | 87.4 | Low |
| Greenfield | 32.4 | 29.8 | 29.8 | 92.0 | Moderate |
| Terre Haute | 33.1 | 30.7 | 32.5 | 96.3 | Moderate |
| Brookville | 34.0 | 31.2 | 32.0 | 97.2 | Moderate |
| Bloomington | 33.5 | 31.8 | 31.2 | 96.5 | Moderate |
| Freelandville | 34.1 | 32.6 | 32.3 | 99.0 | Moderate |
| Vincennes | 35.0 | 30.8 | 33.7 | 99.5 | Moderate |
| Mt. Vernon | 38.8 | 35.2 | 36.8 | 110.8 | High |
![]()
Detecting Wireworms Prior to Planting: It’s Easy and Fun - (Christian Krupke and John Obermeyer)
• Use bait stations to monitor for wireworms.
• Solar and flour bait traps will need to go out very soon.
• The effectiveness of seed-applied insecticides varies depending on wireworm density.
To our surprise, we’ve recently gotten inquires about determining wireworm populations in fields before planting. It likely has to do with the increase of cover crop plantings. Though the jury is still out, cover crop plantings (especially the grass species) may increase wireworm populations over time. There are two trapping methods to determine the potential risk from wireworm to this year’s crop, but they need to be implemented very soon. Wireworms will become active as soil temps warm into the upper 40’s. Both solar and flour bait stations are based on the simple principle that wireworms are attracted to plant volatiles for feeding as soils warm in the spring.
The procedure and materials are quite simple for both “traps” and should require little more than 30 minutes to an hour. Place at least 5 stations in representative areas of a field. For a soil bait station, dig a hole 9 inches in diameter and 6 inches deep. Place a handful each of untreated corn and wheat seed in the hole and cover with soil. Cover the baited area with a piece of black plastic, holding down the sides with soil, and mark the spot with a flag or stake. The black plastic acts as a solar collector, warming the soil surrounding the bait, providing for germination of the corn and wheat seeds. The flour trap is similar, except a cup or two of wheat flour is placed into the hole and then covered with soil. The plastic isn’t necessary, but mark the spot with a flag. Gases given off during germination/decay are attractive to wireworms and they will move to the bait to feed. The bait should be left in the field as long as possible up to the time of planting. At that time, the bait at each station should be dug up and examined for wireworms, this does take some time to do. The bait station will likely smell quite unpleasant at this time. Don’t attempt to re-use this flour for baking!
One or two wireworms found per bait station may warrant the use of higher rates of seed-applied insecticides (i.e., rootworm rate) on the corn seed. Finding numerous wireworms/trap will challenge the efficacy of seed-applied insecticides. In this scenario, granular soil insecticides would afford better protection of the corn seed/seedling especially during slow growing environmental conditions after planting. It is possible this would only be in a portion of the field rather than throughout the field, depending on trap catches. Another, but less desirable option, is to plant soybean where wireworm populations are expected to be heavy. Wireworms feed on soybean seedlings, but the potential loss of thousands of plants/acre is less critical because of the plant’s ability to compensate for thinner stands. Remember, that there is NO rescue treatment for wireworms once the crop has been planted. Happy Scouting!
The materials for a solar bait station
The materials for a solar bait station
Sorting through a dug-up flour trap
![]()
VIDEO: Sorting through a solar bait trap for wireworms
VIDEO: Sorting through a flour bait trap for wireworms
![]()
Handy Bt Trait Table – (Christian Krupke) -
Using Your Water Quality Test Results To Determine How Much AMS To Add To Your Spray Mixture – (Travis Legleiter and Bill Johnson) The Purdue Weed Science Program and Purdue Pesticides Program have urged you to get your spray water quality tested in order to improve the efficiency of the pesticides and adjuvants you are applying. Around this time last year we released a short publication “The Influence of spray water Quality on Herbicide Efficiency”, and at the end of that pub was an equation from North Dakota State University to determine the appropriate amount of AMS to add to spray tank. Apparently we are getting our points across as we have received numerous calls with questions pertaining to the equation and how to use it. The following example will give you a step-by-step breakdown of how to use this equation to your benefit. The first step is to get a water quality test performed by a commercial laboratory that offers analysis of Sodium (Na+), Potassium (K+), Calcium (Ca2+), Magnesium (Mg2+), and Iron (Fe2+) levels. Laboratories typically offer analysis packages that will include measurements of these elements and often more, such as A&L Great Lakes’ irrigation suitability package. Your analysis will likely be reported in either milligrams per liter (Mg/L) or parts per million (ppm); these two units of measurement are interchangeable. Once your results are returned you simply need to plug the ppm or Mg/L values for each element into this equation to determine pounds of AMS needed per 100 gallons of spray mixture: AMS (lbs/100 gal) = 0.005*(Na+) + 0.002*(K+) + 0.009*(Ca2+) + 0.014*(Mg2+) + 0.042*(Fe2+) The following is an example water quality report and calculation of how much AMS needs to be added to the spray mixture using this equation. The analysis results for the five elements from this report are: The equation with input values would be: AMS (lbs/100 gal) = 0.005*(Na+) + 0.002*(K+) + 0.009*(Ca2+) + 0.014*(Mg2+) + 0.042*(Fe2+) Remember from 6th grade math to do the multiplication first, so multiply all values: AMS (lbs/100 gal) = 0.005*(15.7) + 0.002*(1.03) + 0.009*(68.4) + 0.014*(25.21) + 0.042*(0.37) Now do the addition and add all values to get final answer: AMS (lbs/100 gal) = 0.0785 + 0.00206 + 0.6156 + 0.35294 + 0.01554 So the final answer is: AMS (lbs/100 gal) = 1.06464 The value can be rounded up to 1.1 lbs/100 gal for simplicity. So for this particular water source we would add 1.1 pounds of AMS to every 100 gallons of spray mixture. 2013 Popcorn Agri-Chemical Handbook – (Genny Bertalmio, The Popcorn Board) - The 2013 Popcorn Agri-Chemical Handbook is now available at <http://www.popcorn.org/handbook> to ensure everyone in the popcorn industry is informed about products registered for use on popcorn or in popcorn storage facilities. The handbook lists agri-chemicals registered and the regulatory status or special use restrictions, if any. The International Maximum Residue Level (MRL) Database, www.mrldatabase.com, includes popcorn and denotes established levels by the US, Codex, EU and 82 markets. Soon...Very Soon! PURDUE EXTENSION FIELD CROP SPECIALISTS (765) 494-8761 (765) 494-4783
Chris DiFonzo, Michigan State University and Eileen Cullen, University of Wisconsin have revised their wonderfully helpful table of hybrid corn Bt traits. This is a great reference that nicely summarizes the many combinations of Bt traits in the marketplace, including which insects they control and how they differ for refuge area requirements. This one-page publication has gotten kudos from seed dealers and producers alike, and is worth a look. Check out this publication at:
![]()
Example water quality report and calculation of how much AMS needs to be added to the spray mixture using this equation
Parameter
Result
Unit
Reporting Limit
MDL
Analyst
Analysis Date
Method Reference
Alkalinity, CaCO3
317
mg/L
1
SH
3/20/2009
SM-2320B
Conductivity
0.60
mmho/cm
0.01
0.01
SH
3/25/2009
SPA-120.1
pH
7.70
0.01
0.01
SH
3/25/2009
SM(20th)-4500-H+B
Solids, Total Dissolved (est.)
382
mg/L
1.0
SH
3/25/2009
SM(20th)-2510A
Temperature at time of pH reading
14.3
deg. C
0.1
SH
3/25/2009
EPA-150.1
Chloride
6.24
mg/L
3.50
0.67
SH
3/26/2009
EPA-325.2
Nitrogen, Nitrate+Nitrite (as N)
0.17
mg/L
0.05
0.01
SH
3/25/2009
EPA-353.2
Phosphorus, Ortho (as P)
BDL*
mg/L
0.07
0.015
SH
3/24/2009
EPA-365.1 Rev. 2.0
Carbonate, CO3
BDL*
mg/L
1
SH
3/20/2009
EPA-310.1
Bicarbonate, HCO3
385
mg/L
1
SH
3/20/2009
EPA-310.1
Boron
BDL*
mg/L
0.1
MTG
3/23/2009
EPA-200.7
Calcium
68.4
mg/L
1.0
0.26
MTG
3/23/2009
EPA-200.7
Iron
0.37
mg/L
0.08
0.016
MTG
3/23/2009
EPA-200.7
Potassium
1.03
mg/L
0.02
0.03
MTG
3/23/2009
EPA-200.7
Magnesium
25.21
mg/L
0.23
0.05
MTG
3/23/2009
EPA-200.7
Manganese
BDL*
mg/L
0.17
0.03
MTG
3/23/2009
EPA-200.7
Sodium
15.7
mg/L
0.36
0.07
MTG
3/23/2009
EPA-200.7
Sodium (Na+): 15.7 mg/L
Potassium (K+): 1.03 mg/L
Calcium (Ca2+): 68.4 mg/L
Magnesium (Mg2+): 25.21 mg/L
Iron (Fe2+): 0.37 mg/L
* The values in this example are reported in mg/L, but these values could also reported in ppm and the two would be identical in numerical value.![]()
The Popcorn Board urges you to provide the above links to growers or download, print and distribute the updated version of this critical information to them. Contact Genny Bertalmio, +1.312.821.0217 or <gbertalmio@smithbucklin.com>, for further information.
The Popcorn Board accepts voluntary contributions to ensure continued funding of its efforts to provide this important information to the popcorn industry. Checks should be mailed to The Popcorn Board, 8333 Solutions Center, Chicago, IL 60677-8003.
![]()
Entomology: http://extension.entm.purdue.edu/
Steve Yaninek
(765) 494-4554
yaninek@purdue.edu
Head, Dept. of Entomology
Larry Bledsoe
(765) 494-8324
lbledsoe@purdue.edu
Field Crop Insects, CAPS
Jamal Faghihi
(765) 494-5901
jamal@purdue.edu
Nematology
Greg Hunt
(765) 494-4605
hunt@purdue.edu
Beekeeping
Christian Krupke
(765) 494-4912
ckrupke@purdue.edu
Field Crop Insects
Judy Loven
(765) 494-8721
loven@purdue.edu
USDA, APHIS, Animal Damage
Linda J. Mason
(765) 494-4568
lmason@purdue.edu
Food Pest Mgmt. & Stored Grain
John L. Obermeyer
(765) 494-4563
obe@purdue.edu
Field Crop Insects & IPM Specialist
Tammy Luck
FAX: (765) 494-2152 luck@purdue.edu
Administrative Assistant
Agronomy: http://www.ag.purdue.edu/agry/extension
Joe Anderson
(765) 494-4774
janderson@purdue.edu
Head, Dept. of Agronomy
Sylvie Brouder
(765) 496-1489
sbrouder@purdue.edu
Plant Nutrition, Soil Fertility, Water Quality
Jim Camberato
(765) 496-9338
jcambera@purdue.edu
Soil Fertility
Shaun Casteel
(765) 496-3755
scasteel@purdue.edu
Soybean Specialist and Small Grains
Corey Gerber
(765) 496-3755
gerberc@purdue.edu
Director, Diagnostic Training Center
Brad Joern
(765) 494-9767
bjoern@purdue.edu
Soil Fertility, Waste Mgmt.
Keith D. Johnson
(765) 494-4800
johnsonk@purdue.edu
Forages
Charles Mansfield
(812) 888-4311
cmansfie@purdue.edu
Small Grains, Soybean, Corn
Robert L. Nielsen
(765) 494-4802
rnielsen@purdue.edu
Corn, Sorghum, Precision Agriculture
Aaron Patton
(765) 494-9737
ajpatton@purdue.edu
Turfgrass Specialist
Gary Steinhardt
(765) 494-8063
gsteinha@purdue.edu
Soil Mgmt., Tillage, Land Use
Tony Vyn
(765) 496-3757
tvyn@purdue.edu
Soil Mgmt. & Tillage
Terry West
(765) 494-4799
twest@purdue.edu
Soil Mgmt. & Tillage
Lisa Green
FAX: (765) 496-2926 lgreen06@purdue.edu
Extension Secretary
Botany and Plant Pathology: http://www.ag.purdue.edu/btny/Extension
Peter Goldsbrough
(765) 494-4615
goldsbrough@purdue.edu
Head, Dept. of Botany & Plant Pathology
Tom Creswell
(765) 494-7071
cresswell@purdue.edu
Director, Plant & Pest Diagnostic Lab
Dan Egel
(812) 886-0198
egel@purdue.edu
Southwest Purdue Ag Center
Bill Johnson
(765) 494-4656
wgj@purdue.edu
Weed Science
Travis Legleiter
(765) 496-2121
tlegleit@purdue.edu
Weed Science
Gail Ruhl
(765) 494-4641
ruhlg@purdue.edu
Plant & Pest Diagnostic Lab
Fred Whitford
(765) 494-4566
fwhitford@purdue.edu
Purdue Pesticide Programs
Kiersten Wise
(765) 496-2170
kawise@purdue.edu
Diseases of Field Crops
Charles Woloshuk
(765) 494-3450
woloshuk@purdue.edu
Mycotoxins in Corn
Amy Deitrich
(765) 494-9871
FAX: (765)494-0363 amymd@purdue.edu
Extension Assistant/P&PDL Lab Coorinator
Agricultural & Biological Engr.: http://engineering.purdue.edu/ABE/index.html
Bernie Engel
(765) 494-1162
engelb@purdue.edu
Interim Head, Dept. of Ag. & Bio. Engr.
Richard Stroshine
(765) 496-1192
strosh@purdue.edu
Bioenergy
Jane Frankenberger
(765) 494-1194
frankenb@purdue.edu
GIS and Water Quality
Carol Sikler
(765) 494-1174
FAX: (765) 496-1356 sikler@purdue.edu
Extension Assistant