Pest & Crop Newsletter, Entomology Extension, Purdue University
- VIDEO: Scouting Western Bean Cutworm Post-Whorl, Possible But Tedious
- Western Corn Rootworm Beetle Numbers Unimpressive, Likely Good News for Next Year’s Corn
- Black Light Trap Catch Report
- Western Corn Cutworm Adult Pheromone Trap Report
VIDEO: Scouting Western Bean Cutworm Post-Whorl, Possible But Tedious – (Christian Krupke and John Obermeyer)
The vast majority of cornfields in the northwestern counties of Indiana have reached or are past pollination. However, western bean cutworm moths, though beyond peak flight, are still flying and laying eggs. In the last week or so, scouting emphasis has shifted from sampling for egg masses in pre-tassel corn to finding early instar (VERY tiny and elusive) larvae in the ear zone of R1 and R2 corn (refer to Bob Nielsen’s article on corn’s reproductive stages). The following video will hopefully assist you in scouting for and understanding this pest while it attempts to establish in developing corn ears.
Early instar western bean cutworm larva on the silks near a pollen anther
View this video on Western Bean Cutworm Scouting Post-Whorl Corn
![]()
Western Corn Rootworm Beetle Numbers Unimpressive, Likely Good News for Next Year’s Corn - (Christian Krupke and John Obermeyer)
- Emergence continues, but low populations of adults again this year.
- Many fields are pollinating, yet few beetles spotted.
- Fewer beetles = fewer eggs for next year's population.
There is no question that western corn rootworm beetles are low this year, which correlates with the lack of corn lodging reports received… namely zero. The last three springs during egg hatch, frequent rains and saturated soils have dramatically increased larval mortality. However, there may be something else at work here.
Ever-increasing adoption of highly effective Bt hybrids for rootworm control is likely contributing to this decline in population. We know that these hybrids typically cause 90%+ mortality in RW larvae. Contrast that with the years of granular insecticides, which effectively protected the roots and crop, but did not do much to lower populations. The European corn borer (ECB) as a key corn pest may offer some guidance here as well. The Bt toxins for this pest are higher in dose, typically leading to mortality in over 99% of insects that contact it. However, ECB have several dozen other potential hosts, ranging from cattails to many common weeds such as ragweed. Yet, throughout most of the country, ECB is now an afterthought in discussions of key corn pests.
In contrast, corn rootworms are essentially limited to corn as a host – meaning that in areas of high-use Bt-RW corn, essentially every host plant is toxic to them. Could we be seeing the early signs of population-level effects of extensive Bt corn adoption? We don’t know the answer, but it is worth thinking about. It is difficult, if not impossible, to tease apart environmental and agronomic factors in a pest with such a huge geographic range. But it’s reasonable to expect that continued adoption and effectiveness of Bt corn will cause a reduction of WCR populations on an area-wide scale. This makes it even more critical that we continue to be good stewards of this highly-effective technology – plant a refuge!
Consider that fewer beetles will lay fewer eggs for next year. Now is the time when beetle populations can be assessed in pollinating corn and soybean for next year’s rootworm risk where corn will be planted. Pollen from a multitude of weeds (e.g., foxtails, volunteer corn, ragweeds, lambsquarters, pigweeds, etc) will draw them in to feed, potentially leading to unexpected lodging. Investigations in these areas during the next few weeks will help make informed control decisions for next year.
Western corn rootworm beetles love pumpkin flowers for their pollen
![]()
Click here to view the Black Light Trap Catch Report
![]()
Click here to view the Western Bean Cutworm Adult Pheromone Trap Report
Glyphosate Resistant Waterhemp in Indiana – (Chad Brabham and Bill Johnson)
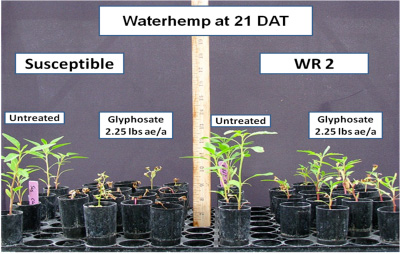
Horseweed, giant ragweed and common ragweed have been confirmed glyphosate-resistant in Indiana and now glyphosate-resistant waterhemp can be added to the list. In the summer of 2009, seeds of two suspected glyphosate-resistant waterhemp populations (WR1 and WR2) were collected from southwest Indiana soybean fields and screened for resistance in greenhouses at Purdue and Southern Illinois University-Carbondale. Plants ranging from 3 to 5 inches in height were sprayed with glyphosate at 0.75 and 2.25 lbs of ae/a. At 21 days after application (DAT) individual plants were rated as dead or alive and percent survival of the two suspect populations were compared to known resistant and susceptible biotypes.
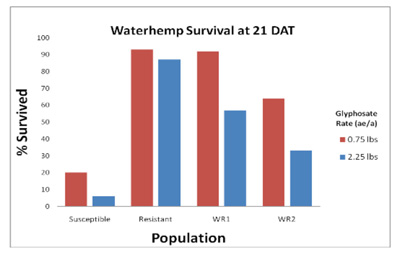
Figure 1. Control of glyphosate susceptible and resistant waterhemp at 21 days after treatment
Glyphosate has generally been an effective herbicide for control of waterhemp. However, control can be variable at times. In the known sensitive population, 0.75 lb ae/A of glyphosate killed 80% of the plants and 94% were killed with 2.25 lbs of glyphosate. The resistant biotype displayed some stunting and chlorosis, but only 10% were killed with either rate of glyphosate and 90% survived. The WR1 population had survival rates of 92% and 57% respectively, with the 0.75 and 2.25 lb rates. Percent survival in the WR2 population was 64% and 33%, respectively. Plants from WR1 and WR2 survived applications that controlled the susceptible biotype indicating glyphosate resistance is present in both populations. Further testing will be performed on future generations to determine the inheritance and level of glyphosate-resistance in these particular populations.
Table 1. Percent survival of waterhemp populations 21 days after glyphosate treatments of 0.75 and 2.25 lbs ae/a
Glyphosate can be used to effectively control glyphosate-susceptible waterhemp, but not glyphosate-resistant populations. Alternative methods will be needed to control glyphosate resistant populations. Researchers in Missouri and Illinois have isolated waterhemp populations that are resistant to glyphosate and PPO (Cobra/Flexstar), PSII (Sencor), and ALS (Classic/FirstRate) inhibiting herbicides. Weed Scientists sarcastically call these populations double, triple, or quadruple stacks!
The bottom line is that we now have glyphosate resistant waterhemp in Indiana. The populations discussed here are in Vigo county in the southwest part of the state. We have significant waterhemp infestations in southern Indiana, northwest Indiana and east central Indiana as well. It would not surprise us if eventually we have glyphosate resistant waterhemp populations in those areas as well if we don’t change management tactics. For more information on control of glyphosate-resistant waterhemp please check out the extension publication ‘Biology and Management of Waterhemp’ at <http://www.glyphosateweedscrops.org/>. We would also like to thank Bryan and Julie Young at SIU for providing seed to use in our greenhouse screening experiments and for running additional screening experiments to confirm that our populations are glyphosate resistant.
Bacterial Stalk Rot of Corn – (Kiersten Wise)
Slimy, discolored corn stalks are being observed in seed and dent corn fields around Indiana. Bacterial stalk rot, can be caused by several species of bacteria, but Erwinia chrysanthemi pv. zeae is likely the cause of many of the symptoms observed in Indiana. This stalk rot can periodically cause problems in Indiana corn, especially when hot, wet weather precedes tasseling.
Symptoms of bacterial stalk rot first appear as tan or brown, water-soaked spots on the leaf sheaths on the internodes of the stalk (Figure 1). Lesions can also form on the leaves, and infected plants wilt and collapse as the infection progresses through the stalks. The pith of infected plants will decay and be mushy or soft. Infected plants are typically scattered throughout a field or in small pockets within a field.
The bacterium that causes this stalk rot survives on residue and infects plants through natural openings on the plant, or wounds caused by heavy rain, high winds, hail damage, or insect feeding. High humidity and high temperatures (85 to 95°F) favor disease development. Plant-to-plant transmission of the bacterium is not common, except where high populations of insects are present.
Management of bacterial stalk rot depends on the type of corn and production system. In hybrid corn fields, cultivation to encourage residue decomposition can help reduce the level of bacteria in affected fields. In seed corn production, avoid excess overhead irrigation, and irrigation from ponds, rivers, or lakes that may harbor bacteria. Kocide 3000 is a fungicide/bactericide that is labeled for bacterial stalk rot management and this chemical may be an option to manage the disease in seed corn production, depending on the size of the affected area within a field.
Figure 1. Tan, water-soaked lesions on leaf sheaths and stalks typical of bacterial stalk rot of corn (Picture courtesy Brian Willard)
Navigating Through the Grain Fill Period – (Bob Nielsen)
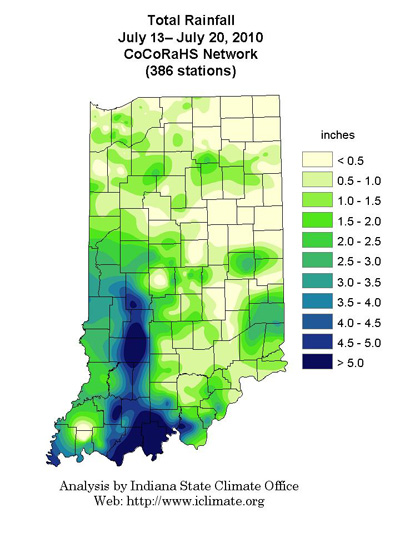
The bulk of Indiana’s corn crop is moving beyond the pollination period and into the early stages of the important grain filling period. Success during the next 45 to 60 days will be measured in terms of kernel set and kernel weight. The degree to which the grain filling period is successful depends primarily on whether the solar-powered photosynthetic factory growers have worked so diligently to construct back in May and June will operate efficiently through early September.
The earliness of planting for many growers, coupled with lengthy periods of above-normal temperatures this year, set the stage for an early maturity date for quite a bit of the Indiana crop. Silking progress to date has been on par with the record-setting earliness of 2004 and certainly reinforces the likelihood for an early maturity to this crop and prospects for more rapid grain drydown in the field prior to harvest than last year’s nightmare.
The fearmongerers among us point to several key factors that could make or break the yield potential of this year’s crop. First of all, it is no secret that there yet exist quite a few “ugly” fields of corn that remain stunted and uneven in their development. The primary culprit was the excessive damage to the root systems from lengthy periods of saturated soils in May and June. Most of these fields will not come close to achieving their maximum yield potential.
Secondly, the frequent and excessive rains back in May and June occurred during the establishment of the root systems of those corn plants. In addition to outright root death, the consequences of extended periods of wet soils include the development of a shallow root system. If the rain “spigot” would shut off for the remainder of the grain fill period, drought-like symptoms would quickly develop and take its toll on kernel survival and kernel weight.
Thirdly, conditions have been favorable for early infection of certain important foliar diseases of corn, including gray leaf spot. Significant loss of photosynthetically-active leaf area due to such diseases, or drought, or nitrogen deficiency, or hail damage during the grain filling period would weaken stalk integrity and increase the plants’ susceptibility to root and stalk rot organisms. This can occur when photosynthetically-challenged plants respond by “cannabilizing” or remobilizing stored carbohydrate reserves from the lower stalks and leaves to the developing grain on the ears. Fewer carbohydrates are available for maintenance of root health and the removal of carbohydrates results in physically weakened lower stalks. Grain yield suffers and harvestability can be compromised.
Fourthly, above-normal temperatures during the grain filling period are not favorable for optimum yield. Excessively warm temperatures encourage a faster grain filling rate per day (good) but a shorter grain filling time period (not good). The abbreviated length of the grain filling period during warm temperature regimes tends to outweigh the benefits of faster daily grain fill rates.
On the positive side............If soil moisture remains adequate and plants remain healthy and temperatures moderate soon, there is tremendous yield potential in some of these fields around the state.
Related Reading
Nielsen, RL (Bob). 2008. Grain Fill Stages in Corn. Corny News Network, Purdue Univ. [online] <http://www.kingcorn.org/news/timeless/GrainFill.html> [URL accessed July 2010].
Nielsen, RL (Bob). 2008. Kernel Set Scuttlebutt. Corny News Network, Purdue Univ. [online] <http://www.kingcorn.org/news/timeless/KernelSet.html> [URL accessed July 2010].
Nielsen, RL (Bob). 2009. Field Drydown of Mature Corn Grain. Corny News Network, Purdue Univ. [online] <http://www.kingcorn.org/news/timeless/GrainDrying.html>. [URL accessed July 2010].
Nielsen, RL (Bob). 2009. Stress During Grain Fill: A Harbinger of Stalk Health Problems Corny News Network, Purdue Univ. [online] <http://www.kingcorn.org/news/timeless/StalkHealth.html>. [URL accessed July 2010].
Wise, Kiersten. 2010. Conditions Favorable for Gray Leaf Spot in Corn. Purdue Pest & Crop Newsletter, Purdue Univ. [online] <http://extension.entm.purdue.edu/pestcrop/2010/issue13/index.html#conditions> [URL accessed July 2010].
![]()
Grain Fill Stages in Corn – (Bob Nielsen)
The grain fill period begins with successful pollination and initiation of kernel development, and ends approximately 60 days later when the kernels are physiologically mature. During grain fill, the developing kernels will be the primary sink for concurrent photosynthate produced by the corn plant. What this means is that the photosynthate demands of the developing kernels will take precedence over that of much of the rest of the plant. In essence, the plant will do all it can to “pump” dry matter into the kernels, sometimes at the expense of the health and maintenance of other plant parts including the roots and lower stalk.
A stress-free grain fill period can maximize the yield potential of a crop, while severe stress during grain fill can cause kernel abortion or lightweight grain and encourage the development of stalk rot. The health of the upper leaf canopy is particularly important for achieving maximum grain filling capacity.
Kernel development proceeds through several relatively distinct stages that were originally described by Ritchie et al. (1993). This article offers a pictorial tour of the grain filling period of a 109 CRM hybrid planted April 18, 2007 at the Purdue Agronomy Farm in west central Indiana.
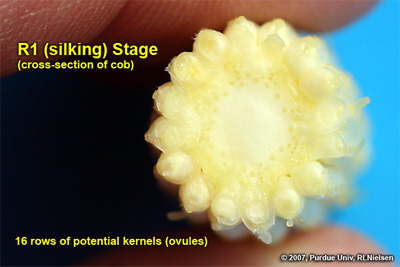
Silking Stage (Growth Stage R1)
Some may argue whether silking should be labeled as a kernel growth stage, but nonetheless silk emergence is technically the first identifiable stage of the reproductive period. Silks remain receptive to pollen grain germination up to 10 days after silk emergence (Nielsen, 2007a). Silk receptivity decreases rapidly after 10 days if pollination has not yet occurred. Natural senescence of silk tissue over time results in collapsed tissue that restricts continued growth of the pollen tube. Silk emergence usually occurs in close synchrony with pollen shed (Nielsen, 2007b), so that duration of silk receptivity is normally not a concern. Failure of silks to emerge in the first place (for example, in response to silkballing or severe drought stress) does not bode well for successful pollination.
Appearance of silks at growth stage R1
Closer view of cob and ovules with silks removed at growth stage R1
Cross-section of cob at growth stage R1
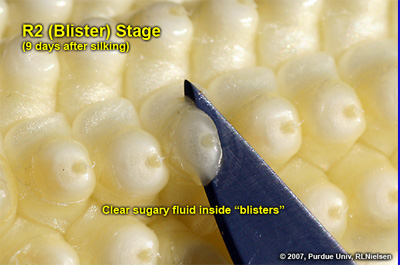
Kernel Blister Stage (Growth Stage R2)
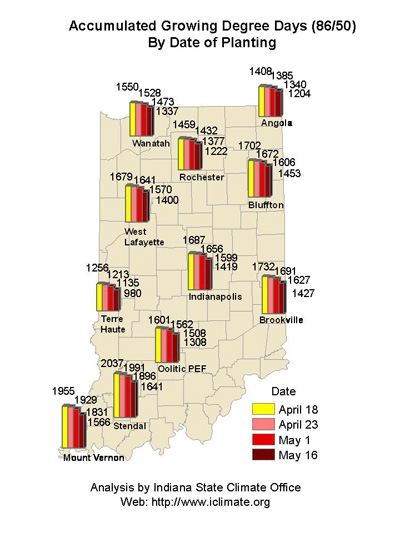
About 10 to 14 days after silking, the developing kernels are whitish “blisters” on the cob and contain abundant clear fluid. The ear silks are mostly brown and drying rapidly. Some starch is beginning to accumulate in the endosperm. The radicle root, coleoptile, and first embryonic leaf have formed in the embryo by the blister stage. Severe stress can easily abort kernels at pre-blister and blister stages. Kernel moisture content is approximately 85 percent. For late April to early May plantings in Indiana, the thermal time from blister stage to physiological maturity is approximately 960 GDDs (Brown, 1999).
Full anther exsertion throughout all tassel branches
Exserted anthers on a tassel
CLoseup of anthers
Tassel Morphology
Approximately 1,000 individual spikelets form on each tassel and each one bears two florets encased in two large glumes. Each floret contains three anthers. An anther and its attached filament comprise the stamen of the male flower. The anthers are those “thingamajigs” that hang from the tassel during pollination. Under a magnifying lens, anthers look somewhat like the double barrel of a shotgun. Do the math and you will realize that an individual tassel produces approximately 6,000 pollen-bearing anthers, although hybrids can vary greatly for this number.
As these florets mature, elongation of the filaments helps exsert the anthers from the glumes. Pollen is dispersed through pores that open at the tips of the anthers. Pollen shed usually begins in the mid-portion of the central tassel spike and then progresses upward, downward and outward over time. Anthers typically emerge from the upper floret of the pair first, while those from lower floret typically emerge later the same day or on following days. Spent anthers eventually drop from the tassel and are sometimes mistaken for the pollen when observed on the leaves or ground.
The yellow or white “dust-like” pollen that falls from a tassel represents millions of individual, nearly microscopic, spherical, yellowish- or whitish translucent pollen grains. Estimates of the total number of pollen grains produced per tassel range from 2 to 25 million. Each pollen grain contains the male genetic material necessary for fertilizing the ovary of one potential kernel.
"Closer view of drying silks at growth stage R2
"Closer view of drying silks at growth stage R2
"Depth of kernels in cross-section of cob at growth stage R2
Kernel Milk Stage (R3)
About 18 to 22 days after silking, the kernels are mostly yellow and contain “milky” white fluid. The milk stage of development is the infamous “roasting ear” stage, that stage where you will find die-hard corn aficionados standing out in their field nibbling on these delectable morsels. Starch continues to accumulate in the endosperm. Endosperm cell division is nearly complete and continued growth is mostly due to cell expansion and starch accumulation. Severe stress can still abort kernels, although not as easily as at the blister stage. Kernel moisture content is approximately 80 percent. For late April to early May plantings in Indiana, the thermal time from milk stage to physiological maturity is approximately 880 GDDs (Brown, 1999).
Ear with husks removed showing kernels and spent silks at growth stage R3
Milky sugary fluid from developing kernel cut with knife at growth stage R3
Depth of kernels in cross-section of cob at growth stage R3
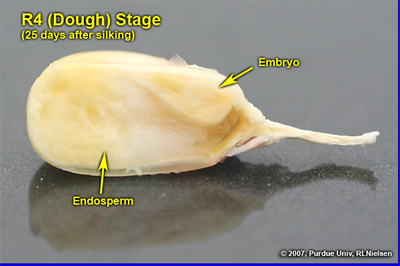
Kernel Dough Stage (R4)
About 24 to 28 days after silking, the kernel’s milky inner fluid is changing to a “doughy” consistency as starch accumulation continues in the endosperm. The shelled cob is now light red or pink. By dough stage, four embryonic leaves have formed and the kernels have reached about 50 percent of their mature dry weight. Kernel moisture content is approximately 70 percent by R4. Kernel abortion is much less likely to occur once kernels have reached early dough stage, but severe stress can continue to affect eventual yield by reducing kernel weight. For late April to early May plantings in Indiana, the thermal time from dough stage to physiological maturity is approximately 670 GDDs (Brown, 1999).
As silks first emerge from the husk, they lengthen as much as 1.5 inches per day for the first day or two, but gradually slow over the next several days. Silk elongation occurs by expansion of existing cells, so elongation rate slows as more and more cells reach maximum size. Elongation of an individual silk stops shortly after pollen is captured, germinates and then penetrates the silk.
Ear with husks removed showing kernels and spent silks at growth stage R4
Depth of kernels in cross-section of cob at growth stage R4. Note the pinkish color developing in the cob tissues
Cross-section of R4 kernel reflecting the conversion of sugary fluids present at R3 in the endosperm to the solid (doughy) starch present at R4
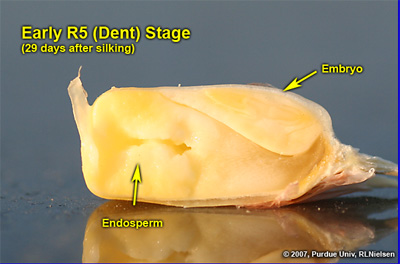
Kernel Dent Stage (R5)
About 35 to 42 days after silking, all or nearly all of the kernels are denting near their crowns. The fifth (and last) embryonic leaf and lateral seminal roots form just prior to the dent stage. Kernel moisture content at the beginning of the dent stage is approximately 55 percent.
A distinct horizontal line appears near the dent end of the kernel and slowly progresses to the tip end of the kernel over the next 3 weeks or so. This line is called the “milk line” and marks the boundary between the liquid (milky) and solid (starchy) areas of the maturing kernels.
For late April to early May plantings in Indiana, the thermal time from full dent (kernel milk line barely visible) to physiological maturity is approximately 350 GDDs (Brown, 1999). Thermal time from the half-milkline stage to physiological maturity for similar planting dates is approximately 280 GDDs. One of the consequences of delayed planting is that thermal time from the dent stage to physiological maturity is shortened, though this may simply reflect a premature maturation of the grain caused by the cumulative effects of shorter daylengths and cooler days in early fall or by outright death of the plants by a killing fall freeze.
Severe stress can continue to limit kernel dry weight accumulation between the dent stage and physiological maturity. Estimated yield loss due to total plant death at full dent is about 40%, while total plant death at half-milkline would decrease yield by about 12% (Carter & Hesterman, 1990).
Kernel apprearance at ealy R5. Approximately 36 kernels per row an about 7 per row and about 7 non-pollinated ovules at tip
Depth of kernels in cross-section of cob at early R5. Kernel milkline not yet visible. Pinkish cob tissue clearly visible
Cross-section of early R5 kernel
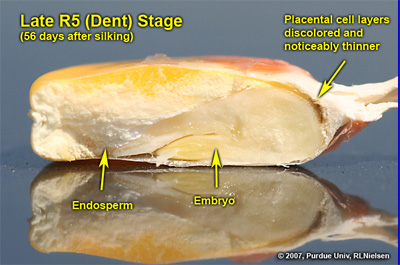
Kernel appearance at late R5. Approximately 42 kernels per row and about 2 non-pollinated ovules at tip
Depth of kernels in cross-section of cob at late R5. Kernel milkline has nearly dissappeared into cob glume tissue
Cross-section of late R5 kernel showing the discolored placental cell layers that have noticeably thinned
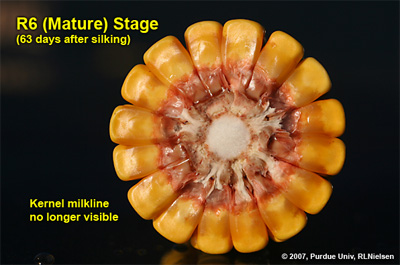
Physiological Maturity (R6)
About 55 to 65 days after silking, kernel dry weight usually reaches its maximum and kernels are said to be physiologically mature and safe from frost. Physiological maturity occurs shortly after the kernel milk line disappears and just before the kernel black layer forms at the tip of the kernels. Severe stress after physiological maturity has little effect on grain yield, unless the integrity of the stalk or ear is compromised (e.g., damage from European corn borer or stalk rots). Kernel moisture content at physiological maturity averages 30 percent, but can vary from 25 to 40 percent grain moisture.
Ear and kernel appearance at growth stage R6 (physiological maturity); about 63 days after mid-silk
Depth of kernels in cross-section of cob at growth stage R6. Kernel milkline has disappeared into cob glume tissue
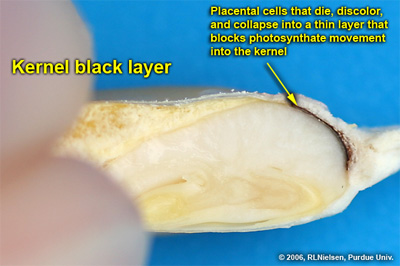
Cross-section of kernel at growth stage R6 depicting the kernel black layer (dead & collapsed placental cells near the tip of the kernel)
Harvest Maturity
While not strictly a stage of grain development, harvest maturity is often defined as that grain moisture content where harvest can occur with minimal kernel damage and mechanical harvest loss. Harvest maturity is usually considered to be near 25 percent grain moisture.
Related References
Brown, Greg A. 1999. Influence of Delayed Planting on Growing Degree Day Requirements of Corn (Zea mays L.) Hybrids During Grain Fill and Maturation. M.S. Thesis, Purdue University.
Carter, P.R. and O.B. Hesterman. 1990. Handling Corn Damaged by Autumn Frost (NCH-57). Purdue Extension. [On-line]. Available at <http://www.ces.purdue.edu/extmedia/NCH/NCH-57.html>. (URL accessed 9/16/08).
Nielsen, R.L. (Bob). 2004. Yield Loss Potential During Grain Fill. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/articles.04/GrainFillStress-0705.html>. (URL accessed 9/16/08).
Nielsen, R.L. (Bob). 2007a. Silk Emergence. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/timeless/Silks.html> (URL accessed 9/16/08).
Nielsen, R.L. (Bob). 2007b. Tassel Emergence & Pollen Shed. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/timeless/Tassels.html> (URL accessed 9/16/08).
Nielsen, R.L. (Bob). 2008. Kernel Set Scuttlebutt. Corny News Network, Purdue Univ. [On-Line]. Available at <http://www.kingcorn.org/news/timeless/KernelSet.html>. (URL accessed 9/16/08).
Ritchie, S.W., J.J. Hanway, and G.O. Benson. 1993. How a Corn Plant Develops. Iowa State Univ. Sp. Rpt. No. 48. [On-Line]. Available at <http://www.extension.iastate.edu/hancock/info/corn.htm>. (URL accessed 9/16/08).